Abstract
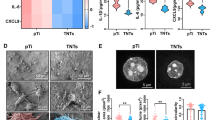
Electrochemically engineered titania (TiO2) nanopores enable tailored cellular function; however, the cellular mechanosensing mechanisms dictating the cell response and soft tissue integration are yet to be elucidated. Here, we report the fabrication of anisotropic TiO2 nanopores with diameters of 46 and 66 nm on microrough titanium (Ti) via electrochemical anodization, towards short- and long-term guidance of human primary gingival fibroblasts (hGFs). Cells on tissue culture plates and bare Ti substrates were used as controls. Notably, we show that nanopores with a diameter of 66 nm induced more mature focal adhesions of vinculin and paxillin at the membrane, encouraged the development of actin fibers at focal adhesion sites, and led to elongated cell and nuclear shape. These topographical-driven changes were attributed to the Ras-related C3 botulinum toxin substrate 1 (Rac 1) GTPase pathway and nuclear localisation of LAMIN A/C and yes-associated protein (YAP) and associated with increased ligament differentiation with elevated expression of the ligament marker Mohawk homeobox (MKX). Study findings reveal that minor tuning of nanopore diameter is a powerful tool to explore intracellular and nuclear mechanotransduction and gain insight into the relationships between nanomaterials and mechanoresponsive cellular elements.

Similar content being viewed by others
References
Kubow, K. E.; Conrad, S. K.; Horwitz, A. R. Matrix microarchitecture and myosin II determine adhesion in 3D matrices. Curr. Biol. 2013, 23, 1607–1619.
Lv, L. W.; Tang, Y. M.; Zhang, P.; Liu, Y. S.; Bai, X. S.; Zhou, Y. S. Biomaterial cues regulate epigenetic state and cell functions—A systematic review. Tissue Eng. Part B Rev. 2018, 24, 112–132.
Ma, C. Y.; Kuzma, M. L.; Bai, X. C.; Yang, J. Biomaterial-based metabolic regulation in regenerative engineering. Adv. Sci. (Weinh.) 2019, 6, 1900819.
Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A. S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572.
Hynes, R. O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219.
Han, P. P.; Gomez, G. A.; Duda, G. N.; Ivanovski, S.; Poh, P. S. P. Scaffold geometry modulation of mechanotransduction and its influence on epigenetics. Acta Biomater., in press, https://doi.org/10.1016/j.actbio.2022.01.020.
Han, P. P.; Vaquette, C.; Abdal-Hay, A.; Ivanovski, S. The mechanosensing and global DNA methylation of human osteoblasts on MEW fibers. Nanomaterials (Basel) 2021, 11, 2943.
Frith, J. E.; Kusuma, G. D.; Carthew, J.; Li, F. Y.; Cloonan, N.; Gomez, G. A.; Cooper-White, J. J. Mechanically-sensitive miRNAs bias human mesenchymal stem cell fate via mTOR signalling. Nat. Commun. 2018, 9, 257.
McMurray, R. J.; Gadegaard, N.; Tsimbouri, P. M.; Burgess, K. V.; McNamara, L. E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R. O. C.; Dalby, M. J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644.
Chen, X. Y.; Lai, N. C. H.; Wei, K. C.; Li, R.; Cui, M.; Yang, B. G.; Wong, S. H. D.; Deng, Y. R.; Li, J. S.; Shuai, X. T. et al. Biomimetic presentation of cryptic ligands via single-chain nanogels for synergistic regulation of stem cells. ACS Nano 2020, 14, 4027–4035.
Seong, H.; Higgins, S. G.; Penders, J.; Armstrong, J. P. K.; Crowder, S. W.; Moore, A. C.; Sero, J. E.; Becce, M.; Stevens, M. M. Size-tunable nanoneedle arrays for influencing stem cell morphology, gene expression, and nuclear membrane curvature. ACS Nano 2020, 14, 5371–5381.
Hansel, C. S.; Crowder, S. W.; Cooper, S.; Gopal, S.; João Pardelha da Cruz, M.; de Oliveira Martins, L.; Keller, D.; Rothery, S.; Becce, M.; Cass, A. E. G. et al. Nanoneedle-mediated stimulation of cell mechanotransduction machinery. ACS Nano 2019, 13, 2913–2926.
Frith, J. E.; Mills, R. J.; Cooper-White, J. J. Lateral spacing of adhesion peptides influences human mesenchymal stem cell behaviour. J. Cell. Sci. 2012, 125, 317–327.
Han, P. P.; Frith, J. E.; Gomez, G. A.; Yap, A. S.; O’Neill, G. M.; Cooper-White, J. J. Five piconewtons: The difference between osteogenic and adipogenic fate choice in human mesenchymal stem cells. ACS Nano 2019, 13, 11129–11143.
Carthew, J.; Abdelmaksoud, H. H.; Hodgson-Garms, M.; Aslanoglou, S.; Ghavamian, S.; Elnathan, R.; Spatz, J. P.; Brugger, J.; Thissen, H.; Voelcker, N. H. et al. Precision surface microtopography regulates cell fate via changes to actomyosin contractility and nuclear architecture. Adv. Sci. (Weinh.) 2021, 8, 2003186.
Matsugaki, A.; Aramoto, G.; Ninomiya, T.; Sawada, H.; Hata, S.; Nakano, T. Abnormal arrangement of a collagen/apatite extracellular matrix orthogonal to osteoblast alignment is constructed by a nanoscale periodic surface structure. Biomaterials 2015, 37, 134–143.
Nakanishi, Y.; Matsugaki, A.; Kawahara, K.; Ninomiya, T.; Sawada, H.; Nakano, T. Unique arrangement of bone matrix orthogonal to osteoblast alignment controlled by Tspan11-mediated focal adhesion assembly. Biomaterials 2019, 209, 103–110.
Xia, J.; Yuan, Y.; Wu, H. Y.; Huang, Y. T.; Weitz, D. A. Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells. Biomaterials 2020, 248, 120014.
Gulati, K.; Kogawa, M.; Maher, S.; Atkins, G.; Findlay, D.; Losic, D. Titania nanotubes for local drug delivery from implant surfaces. In Electrochemically Engineered Nanoporous Materials: Methods, Properties and Applications. Losic, D.; Santos, A., Eds.; Springer: Cham, 2015; pp 307–355.
Bello, D. G.; Fouillen, A.; Badia, A.; Nanci, A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017, 60, 339–349.
Bello, D. G.; Fouillen, A.; Badia, A.; Nanci, A. Nanoporosity stimulates cell spreading and focal adhesion formation in cells with mutated paxillin. ACS Appl. Mater. Interfaces 2020, 12, 14924–14932.
Zheng, H. M.; Tian, Y. J.; Gao, Q.; Yu, Y. J.; Xia, X. Y.; Feng, Z. P.; Dong, F.; Wu, X. D.; Sui, L. Hierarchical micro-nano topography promotes cell adhesion and osteogenic differentiation via integrin α2-PI3K-AKT signaling axis. Front. Bioeng. Biotechnol. 2020, 8, 463.
Haupt, A.; Minc, N. How cells sense their own shape-mechanisms to probe cell geometry and their implications in cellular organization and function. J. Cell Sci. 2018, 131, jcs214015.
Park, J. S.; Moon, D.; Kim, J. S.; Lee, J. S. Cell adhesion and growth on the anodized aluminum oxide membrane. J. Biomed. Nanotechnol. 2016, 12, 575–580.
Koo, S.; Muhammad, R.; Peh, G. S. L.; Mehta, J. S.; Yim, E. K. F. Micro- and nanotopography with extracellular matrix coating modulate human corneal endothelial cell behavior. Acta Biomater. 2014, 10, 1975–1984.
Swaminathan, V.; Waterman, C. M. The molecular clutch model for mechanotransduction evolves. Nat. Cell Biol. 2016, 18, 459–461.
Turner, C. E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236.
Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183.
Cho, S.; Irianto, J.; Discher, D. E. Mechanosensing by the nucleus: From pathways to scaling relationships. J. Cell Biol. 2017, 216, 305–315.
Crowder, S. W.; Leonardo, V.; Whittaker, T.; Papathanasiou, P.; Stevens, M. M. Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell 2016, 18, 39–52.
Worley, K.; Certo, A.; Wan, L. Q. Geometry-force control of stem cell fate. Bionanoscience 2013, 3, 43–51.
DuFort, C. C.; Paszek, M. J.; Weaver, V. M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319.
Bao, L.; Cui, X. J.; Wang, X. Y.; Wu, J. G.; Guo, M. Y.; Yan, N.; Chen, C. Y. Carbon nanotubes promote the development of intestinal organoids through regulating extracellular matrix viscoelasticity and intracellular energy metabolism. ACS Nano 2021, 15, 15858–15873.
Guo, T. Q.; Oztug, N. A. K.; Han, P. P.; Ivanovski, S.; Gulati, K. Old is gold: Electrolyte aging influences the topography, chemistry, and bioactivity of anodized TiO2 nanopores. ACS Appl. Mater. Interfaces 2021, 13, 7897–7912.
Gulati, K.; Moon, H. J.; Kumar, P. T. S.; Han, P. P.; Ivanovski, S. Anodized anisotropic titanium surfaces for enhanced guidance of gingival fibroblasts. Mater. Sci. Eng. C 2020, 112, 110860.
Gulati, K.; Moon, H. J.; Li, T.; Kumar, P. T. S.; Ivanovski, S. Titania nanopores with dual micro-/nano-topography for selective cellular bioactivity. Mater. Sci. Eng. C 2018, 91, 624–630.
Guo, T. Q.; Gulati, K.; Arora, H.; Han, P. P.; Fournier, B.; Ivanovski, S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021, 124, 33–49.
Guo, T. Q.; Gulati, K.; Arora, H.; Han, P. P.; Fournier, B.; Ivanovski, S. Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent. Mater. 2021, 37, 816–831.
Gulati, K.; Li, T.; Ivanovski, S. Consume or conserve: Microroughness of titanium implants toward fabrication of dual micro-nanotopography. ACS Biomater. Sci. Eng. 2018, 4, 3125–3131.
Li, T.; Gulati, K.; Wang, N.; Zhang, Z. T.; Ivanovski, S. Bridging the gap: Optimized fabrication of robust titania nanostructures on complex implant geometries towards clinical translation. J. Colloid Interface Sci. 2018, 529, 452–463.
Gulati, K.; Del Olmo Martinez, R.; Czerwiński, M.; Michalska-Domańska, M. Understanding the influence of electrolyte aging in electrochemical anodization of titanium. Adv. Colloid Interface Sci. 2022, 302, 102615.
Guo, T. Q.; Oztug, N. A. K.; Han, P. P.; Ivanovski, S.; Gulati, K. Untwining the topography-chemistry interdependence to optimize the bioactivity of nano-engineered titanium implants. Appl. Surf. Sci. 2021, 570, 151083.
Ivanovski, S.; Haase, H. R.; Bartold, P. M. Isolation and characterization of fibroblasts derived from regenerating human periodontal defects. Arch. Oral Biol. 2001, 46, 679–688.
Rajpar, I.; Barrett, J. G. Multi-differentiation potential is necessary for optimal tenogenesis of tendon stem cells. Stem Cell Res. Ther. 2020, 11, 152.
Cosgrove, B. D.; Mui, K. L.; Driscoll, T. P.; Caliari, S. R.; Mehta, K. D.; Assoian, R. K.; Burdick, J. A.; Mauck, R. L. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 2016, 15, 1297–1306.
Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758.
Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021, 127, 80–101.
Guo, T. Q.; Ivanovski, S.; Gulati, K. Optimizing titanium implant nano-engineering via anodization. Mater. Des. 2022, 223, 111110.
Jayasree, A.; Gómez-Cerezo, M. N.; Verron, E.; Ivanovski, S.; Gulati, K. Gallium-doped dual micro-nano titanium dental implants towards soft-tissue integration and bactericidal functions. Mater. Today Adv. 2022, 16, 100297.
Arriagada, C.; Silva, P.; Millet, M.; Solano, L.; Moraga, C.; Torres, V. A. Focal adhesion kinase-dependent activation of the early endocytic protein Rab5 is associated with cell migration. J. Biol. Chem. 2019, 294, 12836–12845.
M D Schaller. Paxillin: A focal adhesion-associated adaptor protein. Oncogene. 2001, 20, 6459–6472.
Cabezas, M. D.; Meckes, B.; Mirkin, C. A.; Mrksich, M. Subcellular control over focal adhesion anisotropy, independent of cell morphology, dictates stem cell fate. ACS Nano 2019, 13, 11144–11152.
Hu, Y. L.; Lu, S. Y.; Szeto, K. W.; Sun, J.; Wang, Y. X.; Lasheras, J. C.; Chien, S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci. Rep. 2014, 4, 6024.
Machacek, M.; Hodgson, L.; Welch, C.; Elliott, H.; Pertz, O.; Nalbant, P.; Abell, A.; Johnson, G. L.; Hahn, K. M.; Danuser, G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009, 461, 99–103.
Padmanabhan, J.; Kinser, E. R.; Stalter, M. A.; Duncan-Lewis, C.; Balestrini, J. L.; Sawyer, A. J.; Schroers, J.; Kyriakides, T. R. Engineering cellular response using nanopatterned bulk metallic glass. ACS Nano 2014, 8, 4366–4375.
Thorpe, S. D.; Lee, D. A. Dynamic regulation of nuclear architecture and mechanics—A rheostatic role for the nucleus in tailoring cellular mechanosensitivity. Nucleus 2017, 8, 287–300.
Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770.
Millar, N. L.; Murrell, G. A. C.; McInnes, I. B. Inflammatory mechanisms in tendinopathy-towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122.
Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S. et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542.
Nakamichi, R.; Ito, Y.; Inui, M.; Onizuka, N.; Kayama, T.; Kataoka, K.; Suzuki, H.; Mori, M.; Inagawa, M.; Ichinose, S. et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat. Commun. 2016, 7, 12503.
Gracey, E.; Burssens, A.; Cambré, I.; Schett, G.; Lories, R.; McInnes, I. B.; Asahara, H.; Elewaut, D. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 193–207.
Swift, J.; Ivanovska, I. L.; Buxboim, A.; Harada, T.; Dingal, P. C. D. P.; Pinter, J.; Pajerowski, J. D.; Spinler, K. R.; Shin, J. W.; Tewari, M. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104.
Acknowledgements
T. G. and A. J. are supported by the University of Queensland Graduate School Scholarships (UQGSS). K. G. is supported by the National Health and Medical Research Council (NHMRC) Early Career Fellowship (No. APP1140699). The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, the University of Queensland. This work was performed in part at the Queensland node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano- and microfabrication facilities for Australia’s researchers. This work was supported by International Team for Implantology (ITI) and Australian Dental Research Foundation (ADRF) research grants.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Han, P., Guo, T., Jayasree, A. et al. Tunable nano-engineered anisotropic surface for enhanced mechanotransduction and soft-tissue integration. Nano Res. 16, 7293–7303 (2023). https://doi.org/10.1007/s12274-023-5379-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5379-y