Abstract
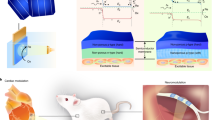
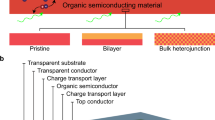
The efficiency of devices for bioelectronic applications, including cell and tissue stimulation, is heavily dependent on the scale and the performance level. With miniaturization of stimulation electrodes, achieving a sufficiently high current pulse to elicit action potentials becomes an issue. Herein we report on our approach of vertically stacking organic p-n junctions to create highly-efficient multilayered organic semiconductor (MOS) photostimulation device. A tandem arrangement substantially increases the photovoltage and charge density without sacrificing lateral area, while not exceeding 200–500 nm of thickness. These devices generate 4 times higher voltages and at least double the charge densities over single p-n junction devices, which allow using lower light intensities for stimulation. MOS devices show an outstanding stability in the electrolyte that is extremely important for forthcoming in vivo experiments. Finally, we have validated MOS devices performance by photostimulating fibroblasts and neuroblasts, and found that using tandem devices leads to more effective action potential generation. As a result, we obtained up to 4 times enhanced effect in cell growth density using 3 p-n layered devices. These results corroborate the conclusion that MOS technology not only can achieve parity with state-of-the-art silicon devices, but also can exceed them in miniaturization and performance for biomedical applications.

Similar content being viewed by others
References
Rand, D.; Jakešová, M.; Lubin, G.; Vėbraitė, I.; David-Pur, M.; Đerek, V.; Cramer, T.; Sariciftci, N. S.; Hanein, Y.; Głowacki, E. D. Direct electrical neurostimulation with organic pigment photocapacitors. Adv. Mater. 2018, 30, 1707292.
Kotov, N. A.; Winter, J. O.; Clements, I. P.; Jan, E.; Timko, B. P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C. M.; Prato, M. et al. Nanomaterials for neural interfaces. Adv. Mater. 2009, 21, 3970–4004.
Pappas, T. C.; Wickramanyake, W. M. S.; Jan, E.; Motamedi, M.; Brodwick, M.; Kotov, N. A. Nanoscale engineering of a cellular interface with semiconductor nanoparticle films for photoelectric stimulation of neurons. Nano Lett. 2007, 7, 513–519.
Santoro, F.; Zhao, W. T.; Joubert, L. M.; Duan, L. T.; Schnitker, J.; Van De Burgt, Y.; Lou, H. Y.; Liu, B. F.; Salleo, A.; Cui, L. F. et al. Revealing the cell—material interface with nanometer resolution by focused ion beam/scanning electron microscopy. ACS Nano 2017, 11, 8320–8328.
Ferro, M. D.; Melosh, N. A. Electronic and ionic materials for neurointerfaces. Adv. Funct. Mater. 2017, 28, 1704335.
Markov, A.; Maybeck, V.; Wolf, N.; Mayer, D.; Offenhäusser, A.; Wördenweber, R. Engineering of neuron growth and enhancing cell-chip communication via mixed SAMs. ACS Appl. Mater. Interfaces 2018, 10, 18507–18514.
Gautam, V.; Rand, D.; Hanein, Y.; Narayan, K. S. A polymer optoelectronic interface provides visual cues to a blind retina. Adv. Mater. 2014, 26, 1751–1756.
Butterwick, A.; Huie, P.; Jones, B. W.; Marc, R. E.; Marmor, M.; Palanker, D. Effect of shape and coating of a subretinal prosthesis on its integration with the retina. Exp. Eye Res. 2009, 88, 22–29.
Maya-Vetencourt, J. F.; Ghezzi, D.; Antognazza, M. R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017, 16, 681–689.
Ghezzi, D.; Antognazza, M. R.; Maccarone, R.; Bellani, S.; Lanzarini, E.; Martino, N.; Mete, M.; Pertile, G.; Bisti, S.; Lanzani, G. et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photonics 2013, 7, 400–406.
Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L. L.; Kamins, T. I.; Galambos, L.; Smith, R.; Harris, J. S.; Sher, A. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photonics 2012, 6, 391–397.
Khodagholy, D.; Gelinas, J. N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G. G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315.
Ahmadraji, T.; Gonzalez-Macia, L.; Ritvonen, T.; Willert, A.; Ylimaula, S.; Donaghy, D.; Tuurala, S.; Suhonen, M.; Smart, D.; Morrin, A. et al. Biomedical diagnostics enabled by integrated organic and printed electronics. Anal. Chem. 2017, 89, 7447–7454.
Yun, S. H.; Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008.
Chuang, A. T.; Margo, C. E.; Greenberg, P. B. Retinal implants: A systematic review. Br. J. Ophthalmol. 2014, 98, 852–856.
Prodanov, D.; Delbeke, J. Mechanical and biological interactions of implants with the brain and their impact on implant design. Front. Neurosci. 2016, 10, 11.
Bareket, L.; Waiskopf, N.; Rand, D.; Lubin, G.; David-Pur, M.; Ben-Dov, J.; Roy, S.; Eleftheriou, C.; Sernagor, E.; Cheshnovsky, O. et al. Semiconductor nanorod-carbon nanotube biomimetic films for wire-free photostimulation of blind retinas. Nano Lett. 2014, 14, 6685–6692.
Zangoli, M.; Di Maria, F.; Zucchetti, E.; Bossio, C.; Antognazza, M. R.; Lanzani, G.; Mazzaro, R.; Corticelli, F.; Baroncini, M.; Barbarella, G. Engineering thiophene-based nanoparticles to induce phototransduction in live cells under illumination. Nanoscale 2017, 9, 9202–9209.
Sytnyk, M.; Jakešová, M.; Litviňuková, M.; Mashkov, O.; Kriegner, D.; Stangl, J.; Nebesářová, J.; Fecher, F. W.; Schöfberger, W.; Sariciftci, N. S. et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals. Nat. Commun. 2017, 8, 91.
Ghezzi, D.; Antognazza, M. R.; Dal Maschio, M.; Lanzarini, E.; Benfenati, F.; Lanzani, G. A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun. 2011, 2, 166.
Abdullaeva, O. S.; Schulz, M.; Balzer, F.; Parisi, J.; Lützen, A.; Dedek, K.; Schiek, M. Photoelectrical stimulation of neuronal cells by an organic semiconductor—electrolyte interface. Langmuir 2016, 32, 8533–8542.
Martino, N.; Feyen, P.; Porro, M.; Bossio, C.; Zucchetti, E.; Ghezzi, D.; Benfenati, F.; Lanzani, G.; Antognazza, M. R. Photothermal cellular stimulation in functional bio-polymer interfaces. Sci. Rep. 2015, 5, 8911.
Shapiro, M. G.; Homma, K.; Villarreal, S.; Richter, C. P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 736.
Jiang, Y. W.; Carvalho-De-Souza, J. L.; Wong, R. C. S.; Luo, Z. Q.; Isheim, D.; Zuo, X. B.; Nicholls, A. W.; Jung, I. W.; Yue, J. P.; Liu, D. J. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 2016, 15, 1023–1030.
Tortiglione, C.; Antognazza, M. R.; Tino, A.; Bossio, C.; Marchesano, V.; Bauduin, A.; Zangoli, M.; Morata, S. V.; Lanzani, G. Semiconducting polymers are light nanotransducers in eyeless animals. Sci. Adv. 2017, 3.
Moros, M.; Lewinska, A.; Onorato, G.; Antognazza, M. R.; Di Francesca, M.; Blasio, M.; Lanzani, G.; Tino, A.; Wnuk, M.; Tortiglione, C. Light-triggered modulation of cell antioxidant defense by polymer semiconducting nanoparticles in a model organism. MRS Commun. 2018, 8, 918–925.
Parameswaran, R.; Carvalho-De-Souza, J. L.; Jiang, Y. W.; Burke, M. J.; Zimmerman, J. F.; Koehler, K.; Phillips, A. W.; Yi, J.; Adams, E. J.; Bezanilla, F. et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 2018, 13, 260–266.
Griffin, M.; Iqbal, S. A.; Sebastian, A.; Colthurst, J.; Bayat, A. Degenerate wave and capacitive coupling increase human msc invasion and proliferation while reducing cytotoxicity in an in vitro wound healing model. PLoS One 2011, 6, e23404.
Matsuki, N.; Takeda, M.; Ishikawa, T.; Kinjo, A.; Hayasaka, T.; Imai, Y.; Yamaguchi, T. Activation of caspases and apoptosis in response to low-voltage electric pulses. Oncol. Rep. 2010, 23, 1425–1433.
O’Hearn, S. F.; Ackerman, B. J.; Mower, M. M. Paced monophasic and biphasic waveforms alter transmembrane potentials and metabolism of human fibroblasts. Biochem. Biophys. Rep. 2016, 8, 249–253.
Horn, R.; Patlak, J. Single channel currents from excised patches of muscle membrane. Proc. Natl. Acad. Sci. USA 1980, 77, 6930–6934.
Chu, X. P.; Papasian, C. J.; Wang, J. Q.; Xiong, Z. G. Modulation of acid-sensing ion channels: Molecular mechanisms and therapeutic potential. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 288–309.
Santoni, G.; Morelli, M. B.; Amantini, C.; Santoni, M.; Nabissi, M.; Marinelli, O.; Santoni, A. “Immuno-transient receptor potential ion channels”: The role in monocyte- and macrophage-mediated inflammatory responses. Front. Immunol. 2018, 9, 1273.
McCaig, C. D.; Rajnicek, A. M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978.
Khitrin, A. K.; Khitrin, K. A.; Model, M. A. A model for membrane potential and intracellular ion distribution. Chem. Phys. Lipids 2014, 184, 76–81.
Ye, H.; Steiger, A. Neuron matters: Electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J. Neuroeng. Rehabil. 2015, 12, 65.
Gerencser, A. A.; Chinopoulos, C.; Birket, M. J.; Jastroch, M.; Vitelli, C.; Nicholls, D. G.; Brand, M. D. Quantitative measurement of mitochondrial membrane potential in cultured cells: Calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 2012, 590, 2845–2871.
Wali, Q.; Elumalai, N. K.; Iqbal, Y.; Uddin, A.; Jose, R. Tandem perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 84, 89–110.
Ameri, T.; Li, N.; Brabec, C. J. Highly efficient organic tandem solar cells: A follow up review. Energy Environ. Sci. 2013, 6, 2390–2413.
Hiramoto, M.; Fujiwara, H.; Yokoyama, M. Three-layered organic solar cell with a photoactive interlayer of codeposited pigments. Appl. Phys. Lett. 1991, 58, 1062–1064.
Hunger, K. Toxicology and toxicological testing of colorants. Rev. Prog. Color. Relat. Top. 2005, 35, 76–89.
Warczak, M.; Gryszel, M.; Jakešová, M.; Đerek, V.; Głowacki, E. D. Organic semiconductor perylenetetracarboxylic diimide (PTCDI) electrodes for electrocatalytic reduction of oxygen to hydrogen peroxide. Chem. Commun. 2018, 54, 1960–1963.
Jacques, S. L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61.
Gerasimenko, A. Y.; Kitsyuk, E.; Kurilova, U. E.; Suetina, I. A.; Russu, L.; Mezentseva, M. V.; Markov, A.; Narovlyansky, A. N.; Kravchenko, S.; Selishchev, S. V. et al. Interfaces based on laser-structured arrays of carbon nanotubes with albumin for electrical stimulation of heart cell growth. Polymers (Basel) 2022, 14, 1866.
Muraoka, R.; Nakano, K.; Kurihara, S.; Yamada, K.; Kawakami, T. Immunohistochemical expression of heat shock proteins in the mouse periodontal tissues due to orthodontic mechanical stress. Eur. J. Med. Res. 2010, 15, 475–482.
Ravikanth, M.; Soujanya, P.; Manjunath, K.; Saraswathi, T.; Ramachandran, C. Heterogenecity of fibroblasts. J. Oral Maxillofac. Pathol. 2011, 15, 247–250.
Hirt, M. N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A. et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161.
Pires, F.; Ferreira, Q.; Rodrigues, C. A. V.; Morgado, J.; Ferreira, F. C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1158–1168.
Głowacki, E. D.; Romanazzi, G.; Yumusak, C.; Coskun, H.; Monkowius, U.; Voss, G.; Burian, M.; Lechner, R. T.; Demitri, N.; Redhammer, G. J. et al. Epindolidiones-versatile and stable hydrogen-bonded pigments for organic field-effect transistors and light-emitting diodes. Adv. Funct. Mater. 2015, 25, 776–787.
Acknowledgements
Research at Sechenov University was funded by the Ministry of Science and Higher Education of the Russian Federation (No. 075-15-2021-596).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Markov, A., Gerasimenko, A., Boromangnaeva, AK. et al. Multilayered organic semiconductors for high performance optoelectronic stimulation of cells. Nano Res. 16, 5809–5816 (2023). https://doi.org/10.1007/s12274-022-5130-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5130-8