Abstract
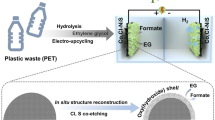
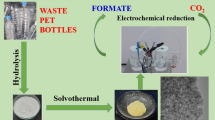
We describe here an electro-reforming strategy to upcycle polyethylene terephthalate (PET) waste with simultaneous hydrogen production by a bifunctional nickel-cobalt nitride nanosheets electrocatalyst. PET plastics are digested in alkaline solution giving an electrochemically active monomer ethylene glycol (EG). The introduction of Co in Co-Ni3N/carbon cloth (CC) promotes the redox behavior of Ni2+/Ni3+, which is beneficial for EG oxidation at an ultra-low potential (1.15 V vs. reversible hydrogen electrode (RHE)) and breaks through the limitation of high catalytic potentials of simple Ni-based electrocatalysts (1.30 V). In PET hydrolysate with Co-Ni3N/CC couples, an integrated EG oxidation-hydrogen production system achieves a current density of 50 mA·cm−2 at a cell voltage of 1.46 V, which is 370 mV lower than the conventional water splitting. The in-situ Raman and Fourier transform infrared (FTIR) spectroscopies and density functional theory (DFT) calculations identify the catalytic mechanism and point to advantages of heterostructure engineering in optimizing adsorption energies and promoting catalytic activities for EG oxidation.

Similar content being viewed by others
References
Geyer, R.; Jambeck, J. R.; Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
Kakadellis, S.; Rosetto, G. Achieving a circular bioeconomy for plastics: Designing plastics for assembly and disassembly is essential to closing the resource loop. Science 2021, 373, 49–50.
Rahimi, A.; García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046.
Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A. A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531.
Nuelle, M. T.; Dekiff, J. H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169.
Achilias, D. S.; Tsintzou, G. P.; Nikolaidis, A. K.; Bikiaris, D. N.; Karayannidis, G. P. Aminolytic depolymerization of poly(ethylene terephthalate) waste in a microwave reactor. Polym. Int. 2011, 60, 500–506.
Thiounn, T.; Smith, R. C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364.
Kim, H. T.; Hee Ryu, M.; Jung, Y. J.; Lim, S.; Song, H. M.; Park, J.; Hwang, S. Y.; Lee, H. S.; Yeon, Y. J.; Sung, B. H. et al. Chemobiological upcycling of poly(ethylene terephthalate) to multifunctional coating materials. ChemSusChem 2021, 14, 4251–4259.
Rorrer, N. A.; Nicholson, S.; Carpenter, A.; Biddy, M. J.; Grundl, N. J.; Beckham, G. T. Combining reclaimed PET with bio-based monomers enables plastics upcycling. Joule 2019, 3, 1006–1027.
Kim, H. T.; Kim, J. K.; Cha, H. G.; Kang, M. J.; Lee, H. S.; Khang, T. U.; Yun, E. J.; Lee, D. H.; Song, B. K.; Park, S. J. et al. Biological valorization of poly(ethylene terephthalate) monomers for upcycling waste PET. ACS Sustainable Chem. Eng. 2019, 7, 19396–19406.
Zhang, F.; Wang, F.; Wei, X. Y.; Yang, Y.; Xu, S. M.; Deng, D. H.; Wang, Y. Z. From trash to treasure: Chemical recycling and upcycling of commodity plastic waste to fuels, high-valued chemicals and advanced materials. J. Energy Chem. 2022, 69, 369–388.
Ügdüler, S.; Van Geem, K. M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394.
Karayannidis, G. P.; Chatziavgoustis, A. P.; Achilias, D. S. Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. 2002, 21, 250–259.
Kao, C. Y.; Cheng, W. H.; Wan, B. Z. Investigation of alkaline hydrolysis of polyethylene terephthalate by differential scanning calorimetry and thermogravimetric analysis. J. Appl. Polym. Sci. 1998, 70, 1939–1945.
Uekert, T.; Kuehnel, M. F.; Wakerley, D. W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857.
Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of nonrecyclable plastic waste over a carbon nitride/nickel phosphide catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210.
Zhu, J.; Hu, L. S.; Zhao, P. X.; Lee, L. Y. S.; Wong, K. Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918.
Yu, Z. Y.; Duan, Y.; Feng, X. Y.; Yu, X. X.; Gao, M. R.; Yu, S. H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100.
Niu, Y. L.; Teng, X.; Gong, S. Q.; Xu, M. Z.; Sun, S. G.; Chen, Z. F. Engineering two-phase bifunctional oxygen electrocatalysts with tunable and synergetic components for flexible Zn-air batteries. Nano-Micro Lett. 2021, 13, 126.
Gong, S. Q.; Niu, Y. L.; Teng, X.; Liu, X.; Xu, M. Z.; Xu, C.; Meyer, T. J.; Chen, Z. F. Visible light-driven, selective CO2 reduction in water by In-doped Mo2C based on defect engineering. Appl. Catal. B: Environ. 2022, 310, 121333.
Han, C.; Li, W. J.; Wang, J. Z.; Huang, Z. G. Boron leaching: Creating vacancy-rich Ni for enhanced hydrogen evolution. Nano Res. 2022, 15, 1868–1873.
Zhang, Y. C.; Han, C. D.; Gao, J.; Pan, L.; Wu, J. T.; Zhu, X. D.; Zou, J. J. NiCo-based electrocatalysts for the alkaline oxygen evolution reaction: A review. ACS Catal. 2021, 11, 12485–12509.
Lei, Y. T.; Zhang, L. L.; Xu, W. J.; Xiong, C. L.; Chen, W. X.; Xiang, X.; Zhang, B.; Shang, H. S. Carbon-supported high-entropy Co-Zn-Cd-Cu-Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Res. 2022, 15, 6054–6061.
Wang, S. C.; Liu, B. Y.; Wang, X.; Zhang, Y. J.; Huang, W. Nanoporous MoO3−x/BiVO4 photoanodes promoting charge separation for efficient photoelectrochemical water splitting. Nano Res. 2022, 15, 7026–7033.
Wang, Z. Y.; Xu, L.; Huang, F. Z.; Qu, L. B.; Li, J. T.; Owusu, K. A.; Liu, Z. A.; Lin, Z. F.; Xiang, B. H.; Liu, X. et al. Copper-nickel nitride nanosheets as efficient bifunctional catalysts for hydrazine-assisted electrolytic hydrogen production. Adv. Energy Mater. 2019, 9, 1900390.
Zheng, S. Y.; Qin, H. Y.; Cao, X. J.; Wang, T. Z.; Lu, W. B.; Jiao, L. F. Electron modulation of cobalt carbonate hydroxide by Mo doping for urea-assisted hydrogen production. J. Energy Chem. 2022, 70, 258–265.
Li, M.; Deng, X. H.; Liang, Y.; Xiang, K.; Wu, D.; Zhao, B.; Yang, H. P.; Luo, J. L.; Fu, X. Z. CoxP@NiCo-LDH heteronanosheet arrays as efficient bifunctional electrocatalysts for co-generation of value-added formate and hydrogen with less-energy consumption. J. Energy Chem. 2020, 50, 314–323.
Gao, L. F.; Liu, Z. B.; Ma, J. L.; Zhong, L. J.; Song, Z. Q.; Xu, J. N.; Gan, S. Y.; Han, D. X.; Niu, L. NiSe@NiOx core-shell nanowires as a non-precious electrocatalyst for upgrading 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid. Appl. Catal. B: Environ. 2020, 261, 118235.
Liu, W. J.; Xu, Z. R.; Zhao, D. T.; Pan, X. Q.; Li, H. C.; Hu, X.; Fan, Z. Y.; Wang, W. K.; Zhao, G. H.; Jin, S. et al. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11, 265.
Li, Y.; Wei, X. F.; Chen, L. S.; Shi, J. L. Electrocatalytic hydrogen production trilogy. Angew. Chem., Int. Ed. 2021, 60, 19550–19571.
Li, L. G.; Wang, P. T.; Shao, Q.; Huang, X. Q. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106.
Liu, X.; Wang, K. K.; Zhou, L. M.; Pu, H. K.; Zhang, T.; Jia, J.; Deng, Y. J. Shape-controlled synthesis of concave pt and willow-like Pt nanocatalysts via electrodeposition with hydrogen adsorption/desorption and investigation of their electrocatalytic performances toward ethanol oxidation reaction. ACS Sustainable Chem. Eng. 2020, 8, 6449–6457.
Ji, L. L.; Wang, J. Y.; Teng, X.; Meyer, T. J.; Chen, Z. F. CoP nanoframes as bifunctional electrocatalysts for efficient overall water splitting. ACS Catal. 2020, 10, 412–419.
Mondal, B.; Karjule, N.; Singh, C.; Shimoni, R.; Volokh, M.; Hod, I.; Shalom, M. Unraveling the mechanisms of electrocatalytic oxygenation and dehydrogenation of organic molecules to value-added chemicals over a Ni-Fe oxide catalyst. Adv. Energy Mater. 2021, 11, 2101858.
Li, J. N.; Li, J. L.; Liu, T.; Chen, L.; Li, Y. F.; Wang, H. L.; Chen, X. R.; Gong, M.; Liu, Z. P.; Yang, X. J. Deciphering and suppressing over-oxidized nitrogen in nickel-catalyzed urea electrolysis. Angew. Chem., Int. Ed. 2021, 60, 26656–26662.
Ni, S.; Qu, H. N.; Xu, Z. H.; Zhu, X. Y.; Xing, H. F.; Wang, L.; Yu, J. M.; Liu, H. Z.; Chen, C. M.; Yang, L. R. Interfacial engineering of the NiSe2/FeSe2 p-p heterojunction for promoting oxygen evolution reaction and electrocatalytic urea oxidation. Appl. Catal. B: Environ. 2021, 299, 120638.
Geng, S. K.; Zheng, Y.; Li, S. Q.; Su, H.; Zhao, X.; Hu, J.; Shu, H. B.; Jaroniec, M.; Chen, P.; Liu, Q. H. et al. Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst. Nat. Energy 2021, 6, 904–912.
Wang, L. P.; Zhu, Y. J.; Wen, Y. Z.; Li, S. Y.; Cui, C. Y.; Ni, F. L.; Liu, Y. X.; Lin, H. P.; Li, Y. Y.; Peng, H. S. et al. Regulating the local charge distribution of Ni active sites for the urea oxidation reaction. Angew. Chem., Int. Ed. 2021, 60, 10577–10582.
Liu, X.; Fang, Z. Y.; Teng, X.; Niu, Y. L.; Gong, S. Q.; Chen, W.; Meyer, T. J.; Chen, Z. Paired formate and H2 productions via efficient bifunctional Ni-Mo nitride nanowire electrocatalysts. J. Energy Chem. 2022, 72, 432–441.
Barwe, S.; Weidner, J.; Cychy, S.; Morales, D. M.; Dieckhöfer, S.; Hiltrop, D.; Masa, J.; Muhler, M.; Schuhmann, W. Electrocatalytic oxidation of 5-(hydroxymethyl)furfural using high-surface-area nickel boride. Angew. Chem., Int. Ed. 2018, 57, 11460–11464.
Li, M.; Deng, X. H.; Xiang, K.; Liang, Y.; Zhao, B.; Hao, J.; Luo, J. L.; Fu, X. Z. Value-added formate production from selective methanol oxidation as anodic reaction to enhance electrochemical hydrogen cogeneration. ChemSusChem 2020, 13, 914–921.
Li, Y.; Wei, X. F.; Chen, L. S.; Shi, J. L.; He, M. Y. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat. Commun. 2019, 10, 5335.
Sha, L. N.; Yin, J. L.; Ye, K.; Wang, G.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G. L.; Cao, D. X. The construction of self-supported thorny leaf-like nickel-cobalt bimetal phosphides as efficient bifunctional electrocatalysts for urea electrolysis. J. Mater. Chem. A 2019, 7, 9078–9085.
Gao, X. R.; Yu, Y.; Liang, Q. R.; Pang, Y. J.; Miao, L. Q.; Liu, X. M.; Kou, Z. K.; He, J. Q.; Pennycook, S. J.; Mu, S. C. et al. Surface nitridation of nickel-cobalt alloy nanocactoids raises the performance of water oxidation and splitting. Appl. Catal. B: Environ. 2020, 270, 118889.
Zhu, C. R.; Wang, A. L.; Xiao, W.; Chao, D. L.; Zhang, X.; Tiep, N. H.; Chen, S.; Kang, J. N.; Wang, X.; Ding, J. et al. In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting. Adv. Mater. 2018, 30, 1705516.
Chen, W.; Xu, L. T.; Zhu, X. R.; Huang, Y. C.; Zhou, W.; Wang, D. D.; Zhou, Y. Y.; Du, S. Q.; Li, Q. L.; Xie, C. et al. Unveiling the electrooxidation of urea: Intramolecular coupling of the N-N bond. Angew. Chem., Int. Ed. 2021, 60, 7297–7307.
Zhao, B.; Liu, J. W.; Wang, X. W.; Xu, C. Y.; Sui, P.; Feng, R. F.; Wang, L.; Zhang, J. J.; Luo, J. L.; Fu, X. Z. CO2-emission-free electrocatalytic CH3OH selective upgrading with high productivity at large current densities for energy saved hydrogen co-generation. Nano Energy 2021, 80, 105530.
Antolini, E.; Gonzalez, E. R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450.
Li, Y.; Wei, X. F.; Han, S. H.; Chen, L. S.; Shi, J. L. MnO2 electrocatalysts coordinating alcohol oxidation for ultra-durable hydrogen and chemical productions in acidic solutions. Angew. Chem., Int. Ed. 2021, 60, 21464–21472.
Gomes, J. F.; Garcia, A. C.; Gasparotto, L. H. S.; de Souza, N. E.; Ferreira, E. B.; Pires, C.; Tremiliosi-Filho, G. Influence of silver on the glycerol electro-oxidation over AuAg/C catalysts in alkaline medium: A cyclic voltammetry and in situ FTIR spectroscopy study. Electrochim. Acta 2014, 144, 361–368.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22072107 and 21872105), the Science & Technology Commission of Shanghai Municipality (No. 19DZ2271500), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, X., Fang, Z., Xiong, D. et al. Upcycling PET in parallel with energy-saving H2 production via bifunctional nickel-cobalt nitride nanosheets. Nano Res. 16, 4625–4633 (2023). https://doi.org/10.1007/s12274-022-5085-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5085-9