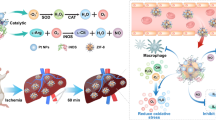
Abstract
Liver transplantation (LT), an ultimate and vital method for treating end-stage liver disease, is often accompanied by ischemia-reperfusion injury (IRI) resulting from warm or cold ischemia of the donor liver. Organ protection techniques are used to improve the quality of liver grafts (from retrieval to implantation). Reactive oxygen species (ROS) cause oxidative stress, which is considered a crucial factor in IRI after LT. Nano antioxidants capable of scavenging ROS alleviate IRI in multiple types of organs and tissues. In this study, we synthesized ceria nanoparticles (NPs) with antioxidant properties using a pyrolysis method and covered them with phospholipid-polyethylene glycol to improve their biocompatibility in vivo. We investigated the potential organ-protective effect of ceria NPs and the underlying mechanisms. Ceria NPs promoted liver function recovery after LT by attenuating IRI in liver grafts in vivo. The protective effect of ceria NPs on liver grafts was investigated by applying hypothermic oxygenated machine perfusion ex vivo. Ceria NPs attenuated hypoxia reoxygenation- or H2O2-induced hepatocyte injury by enhancing mitochondrial activity and ROS scavenging in vitro. These effects may be associated with the activation of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1)/heme oxygenase 1 (HO-1) signaling pathway. In conclusion, ceria NPs may serve as a promising antioxidant agent for the treatment of hepatic IRI after LT.

Similar content being viewed by others
References
Bao, Q. Q.; Hu, P.; Xu, Y. Y.; Cheng, T. S.; Wei, C. Y.; Pan, L. M.; Shi, J. L. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 2018, 12, 6794–6805.
Trapero-Marugán, M.; Little, E. C.; Berenguer, M. Stretching the boundaries for liver transplant in the 21st century. Lancet Gastroenterol. Hepatol. 2018, 3, 803–811.
Liu, Y.; Lu, T. F.; Zhang, C.; Xu, J.; Xue, Z. Z.; Busuttil, R. W.; Xu, N.; Xia, Q.; Kupiec-Weglinski, J. W.; Ji, H. F. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J. Hepatol. 2019, 71, 719–730.
van Rijn, R.; Schurink, I. J.; de Vries, Y.; van den Berg, A. P.; Cerisuelo, M. C.; Murad, S. D.; Erdmann, J. I.; Gilbo, N.; de Haas, R. J.; Heaton, N. et al. Hypothermic machine perfusion in liver transplantation—A randomized trial. N. Engl. J. Med. 2021, 384, 1391–1401.
Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Delgado, T. C.; Simón, J.; Ramos, D. F.; Barriales, D.; Cornide, M. E.; Jiménez, M.; Pérez-Redondo, M.; Lachiondo-Ortega, S. et al. Mitochondrial bioenergetics boost macrophage activation, promoting liver regeneration in metabolically compromised animals. Hepatology 2022, 75, 550–566.
Ali, A.; Wang, A. Z.; Ribeiro, R. V. P.; Beroncal, E. L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E. et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601.
Schlegel, A.; Porte, R. J.; Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J. Hepatol. 2022, 76, 1330–1347.
Giraud, S.; Kerforne, T.; Zely, J.; Ameteau, V.; Couturier, P.; Tauc, M.; Hauet, T. The inhibition of eIF5A hypusination by GC7, a preconditioning protocol to prevent brain death-induced renal injuries in a preclinical porcine kidney transplantation model. Am. J. Transplant. 2020, 20, 3326–3340.
Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P. A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91.
Martins, R. M.; Teodoro, J. S.; Furtado, E.; Rolo, A. P.; Palmeira, C. M.; Tralhão, J. G. Recent insights into mitochondrial targeting strategies in liver transplantation. Int. J. Med. Sci. 2018, 15, 248–256.
Zhang, H. F.; Yan, Q.; Wang, X.; Chen, X.; Chen, Y.; Du, J.; Chen, L. J. The role of mitochondria in liver ischemia-reperfusion injury: From aspects of mitochondrial oxidative stress, mitochondrial fission, mitochondrial membrane permeable transport pore formation, mitophagy, and mitochondria-related protective measures. Oxid. Med. Cell. Longev. 2021, 2021, 6670579.
Ko, S. F.; Chen, Y. L.; Sung, P. H.; Chiang, J. Y.; Chu, Y. C.; Huang, C. C.; Huang, C. R.; Yip, H. K. Hepatic 31P-mggnttic resonance spectroscopy identified the impact of melatonin-pretreated mitochondria in acute liver ischaemia-reperfusion injury. J. Cell. Mol. Med. 2020, 24, 10088–10099.
Sha, Z. L.; Yang, Y. J.; Liu, R. L.; Bao, H. L.; Song, S. H.; Dong, J. F.; Guo, M.; Zhao, Y. Y.; Liu, H.; Ding, G. S. Hepatic ischemia-reperfusion injury in mice was alleviated by rac1 inhibition—More than just ROS-inhibition. J. Clin. Transl. Hepatol. 2022, 10, 42–52.
Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772.
Cannistrà, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70.
Li, S. X.; Bennett, Z. T.; Sumer, B. D.; Gao, J. M. Nano-immune-engineering approaches to advance cancer immunotherapy: Lessons from ultra-pH-sensitive nanoparticles. Acc. Chem. Res. 2020, 53, 2546–2557.
Zhang, T. R.; Tai, Z. G.; Cui, Z.; Chai, R. R.; Zhu, Q. G.; Chen, Z. J. Nano-engineered immune cells as “guided missiles” for cancer therapy. J. Control. Release 2022, 341, 60–79.
Cui, J. J.; Qin, L. F.; Zhang, J. W.; Abrahimi, P.; Li, H.; Li, G. X.; Tietjen, G. T.; Tellides, G.; Pober, J. S.; Mark Saltzman, W. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat. Commun. 2017, 8, 191.
Soh, M.; Kang, D. W.; Jeong, H. G.; Kim, D.; Kim, D. Y.; Yang, W.; Song, C.; Baik, S.; Choi, I. Y.; Ki, S. K. et al. Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem., Int. Ed. 2017, 56, 11399–11403.
Yu, H.; Jin, F. Y.; Liu, D.; Shu, G. F.; Wang, X. J.; Qi, J.; Sun, M. C.; Yang, P.; Jiang, S. P.; Ying, X. Y. et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357.
Kwon, H. J.; Cha, M. Y.; Kim, D.; Kim, D. K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 2016, 10, 2860–2870.
Kang, D. W.; Kim, C. K.; Jeong, H. G.; Soh, M.; Kim, T.; Choi, I. Y.; Ki, S. K.; Kim, D. Y.; Yang, W.; Hyeon, T. et al. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017, 10, 2743–2760.
Ni, D. L.; Wei, H.; Chen, W. Y.; Bao, Q. Q.; Rosenkrans, Z. T.; Barnhart, T. E.; Ferreira, C. A.; Wang, Y. P.; Yao, H. L.; Sun, T. W. et al. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: The perfect imperfection. Adv. Mater. 2019, 31, 1902956.
Wu, X.; Liu, S. Y.; Zhu, H. H.; Ma, Z. L.; Dai, X. H.; Liu, W. W. Scavenging ROS to alleviate acute liver injury by ZnO-NiO@COOH. Adv. Sci (Weinh.) 2022, 9, 2103982.
Fu, S. Y.; Chen, H. L.; Yang, W. T.; Xia, X. H.; Zhao, S.; Xu, X. N.; Ai, P.; Cai, Q. Y.; Li, X. Y.; Wang, Y. et al. ROS-targeted depression therapy via BSA-incubated ceria nanoclusters. Nano Lett. 2022, 22, 4519–4527.
Wang, M. L.; Zeng, F.; Ning, F. L.; Wang, Y. H.; Zhou, S. L.; He, J. Q.; Li, C.; Wang, C.; Sun, X. L.; Zhang, D. L. et al. Ceria nanoparticles ameliorate renal fibrosis by modulating the balance between oxidative phosphorylation and aerobic glycolysis. J. Nanobiotechnology. 2022, 20, 3.
Tanimoto, T.; Parseghian, M. H.; Nakahara, T.; Kawai, H.; Narula, N.; Kim, D.; Nishimura, R.; Weisbart, R. H.; Chan, G.; Richieri, R. A. et al. Cardioprotective effects of HSP72 administration on ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2017, 70, 1479–1492.
Bian, Y. Q.; Chen, Y.; Wang, X. F.; Cui, G. Z.; Ung, C. O. L.; Lu, J. H.; Cong, W. H.; Tang, B. Q.; Lee, S. M. Y. Oxyphylla A ameliorates cognitive deficits and alleviates neuropathology via the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways in vitro and in vivo murine models of Alzheimer’s disease. J. Adv. Res. 2021, 34, 1–12.
da Silva, R. T.; Machado, I. F.; Teodoro, J. S.; Panisello-Rosello, A.; Roselló-Catafau, J.; Rolo, A. P.; Palmeira, C. M. PEG35 as a preconditioning agent against hypoxia/reoxygenation injury. Int. J. Mol. Sci. 2022, 23, 1156.
Zeng, J.; Zhu, L.; Liu, J.; Zhu, T.; Xie, Z. H.; Sun, X. O.; Zhang, H. Metformin protects against oxidative stress injury induced by ischemia/reperfusion via regulation of the lncRNA-H19/miR-148a-3p/Rock2 axis. Oxid. Med. Cell. Longev. 2019, 2019, 8768327.
Chen, C. J.; Yao, W. F.; Wu, S.; Zhou, S. L.; Ge, M.; Gu, Y.; Li, X.; Chen, G. H.; Bellanti, J. A.; Zheng, S. G. et al. Crosstalk between connexin32 and mitochondrial apoptotic signaling pathway plays a pivotal role in renal ischemia reperfusion-induced acute kidney injury. Antioxid. Redox Signal. 2019, 30, 1521–1538.
Zhang, H. L.; Yang, N. Z.; He, H. Y.; Chai, J. W.; Cheng, X. X.; Zhao, H. H.; Zhou, D. H.; Teng, T. M.; Kong, X. R.; Yang, Q. et al. The zinc transporter ZIP7 (Slc39a7) controls myocardial reperfusion injury by regulating mitophagy. Basic Res. Cardiol. 2021, 116, 54.
Schlegel, A.; Graf, R.; Clavien, P. A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J. Hepatol. 2013, 59, 984–991.
Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M. T. P. R.; Isaac, J. R.; Clavien, P. A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57.
Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P. A.; Dutkowski, P. Novel real-time prediction of liver graft function during hypothermic oxygenated machine perfusion before liver transplantation. Ann. Surg. 2019, 270, 783–790.
Lu, T. Y.; Zhang, J. B.; Cai, J. Y.; Xiao, J. Q.; Sui, X.; Yuan, X. F.; Li, R.; Li, Y.; Yao, J.; Lv, G. et al. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials 2022, 284, 121486.
Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting marginal organs in the era of modern machine perfusion and advanced organ monitoring. Front. Immunol. 2020, 11, 631.
Wang, L. H.; Li, J.; He, S.; Liu, Y.; Chen, H. T.; He, S. J.; Yin, M. X.; Zou, D. W.; Chen, S. R.; Luo, T. et al. Resolving the graft ischemia-reperfusion injury during liver transplantation at the single cell resolution. Cell Death Dis. 2021, 12, 589.
Javaherian, K.; Liu, J. F.; Wang, J. C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 1978, 199, 1345–1346.
Scaffidi, P.; Misteli, T.; Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195.
Tsung, A.; Klune, J. R.; Zhang, X. H.; Jeyabalan, G.; Cao, Z. X.; Peng, X. M.; Stolz, D. B.; Geller, D. A.; Rosengart, M. R.; Billiar, T. R. HMGB1 release induced by liver ischemia involves Toll-like receptor 4-dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007, 204, 2913–2923.
Shekaftik, S. O.; Nasirzadeh, N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: A systematic review. Nanotoxicology 2021, 15, 850–864.
Pan, C. Y.; Yu, J. X.; Yao, Q.; Lin, N.; Lu, Z. P.; Zhang, Y.; Zhao, S. S.; Wang, Z. X.; Lei, X. N.; Tian, Y. et al. Prenatal neonicotinoid insecticides exposure, oxidative stress, and birth outcomes. Environ. Int. 2022, 163, 107180.
Ouzounidis, N.; Giakoustidis, A.; Poutahidis, T.; Angelopoulou, K.; Iliadis, S.; Chatzigiagkos, A.; Zacharioudaki, A.; Angelopoulos, S.; Papalois, A.; Papanikolaou, V. et al. Interleukin 18 binding protein ameliorates ischemia/reperfusion-induced hepatic injury in mice. Liver Transpl. 2016, 22, 237–246.
Zhang, S.; Cao, Y.; Xu, B.; Zhang, H.; Zhang, S. T.; Sun, J.; Tang, Y.; Wang, Y. H. An antioxidant nanodrug protects against hepatic ischemia-reperfusion injury by attenuating oxidative stress and inflammation. J. Mater. Chem. B, in press, https://doi.org/10.1039/D1TB02689E.
Bai, H.; Wen, J.; Gong, J. P.; Wu, H.; Yuan, F. C.; Cao, D.; Wu, Y. K.; Lai, X.; Wang, M. H. Blockade of the Notch1/Jagged1 pathway in Kupffer cells aggravates ischemia-reperfusion injury of orthotopic liver transplantation in mice. Autoimmunity 2019, 52, 176–184.
Sadler, J. E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 1998, 67, 395–424.
Yang, L.; Cao, H.; Sun, D.; Hou, B.; Lin, L.; Shen, Z. Y.; Song, H. L. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality—The important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020, 381, 239–254.
Chen, H.; Zheng, H. Z.; Li, T. J.; Jiang, Q. H.; Liu, S. L.; Zhou, X. X.; Ding, Y. T.; Xiang, X. W. Protective effect of oyster peptides derived from Crassostrea gigas on intestinal oxidative damage induced by cyclophosphamide in mice mediated through Nrf2-Keap1 signaling pathway. Front. Nutr. 2022, 9, 888960.
Liou, S. F.; Nguyen, T. T. N.; Hsu, J. H.; Sulistyowati, E.; Huang, S. E.; Wu, B. N.; Lin, M. C.; Yeh, J. L. The preventive effects of xanthohumol on vascular calcification induced by vitamin D3 plus nicotine. Antioxidants (Basel) 2020, 6, 956.
Acknowledgements
This study was supported by Public Projects of Zhejiang Province (No. LGF21H030006), Major Science and Technology Projects of Hainan Province (No. ZDKJ2019009), the Zhejiang Provincial Natural Science Foundation of China (No. LZ21H180001), a Research Project of Jinan Microecological Biomedicine Shandong Laboratory (Nos. JNL-2022002A, JNL-2022007B, and JNL-2022023C), and the National Natural Science Foundation of China (No. 82000618).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Qiao, Y., Li, J., Bian, S. et al. Application of biocompatible custom ceria nanoparticles in improving the quality of liver grafts for transplantation. Nano Res. 16, 5176–5188 (2023). https://doi.org/10.1007/s12274-022-5071-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5071-2