Abstract
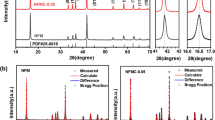
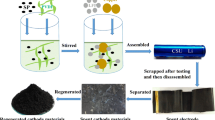
Rechargeable aluminum batteries (RABs) are a popular energy storage device because of its safety and environmental protection. As cathode materials of RABs, transition metal oxide, sulfide, and selenide have become the research hotspot. In this work, we have successfully prepared CuO, Cu1.8S, and Cu1.8Se electrode materials. Among them, although Cu1.8Se had a relatively higher initial discharge capacity, all of these products had severe capacity degradation in terms of cycling and rate performance. Furthermore, for solving the problem of capacity decline, CMK-3 modified separator was used to make the Cu1.8Se cathode material more stable, thus improving cycling and rate performance. It can be confirmed by ex situ X-ray photoelectron spectroscopy (XPS) that both Cu and Se elements underwent reversible redox reactions during the charging/discharging process. Density functional theory was implemented to study the energy storage mechanism of CumX (X = O, S, Se). The results showed that Cu1.8S and Cu1.8Se mainly relied on AlCl4− for energy storage, and the intercalation/de-intercalation of Al3+ occurred during the charge/discharge process in CuO material. Consequently, the optimized Cu1.8Se/CMK-3@GF/C/Al revealed an outstanding rate capability (977.83 mAh·g−1 at 0.5 A·g−1) and long cyclic stability (retention of 478.77 mAh·g−1 after 500 cycles at 1.0 A·g−1). Compared to previously reported cathode materials of RABs, this type of battery displays great superiority in terms of rate and cycling stability. This research also provides a novel approach to suppress the shuttle effect of active species for advanced clean energy devices.

Similar content being viewed by others
References
Armand, M.; Tarascon, J. M. Building better batteries. Nature 2008, 451, 652–657.
Goodenough, J. B.; Park, K. S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
Yang, Z.; Zhong, J. J.; Feng, J. M.; Li, J. L.; Kang, F. Y. Highly reversible anion redox of manganese-based cathode material realized by electrochemical ion exchange for lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2103594.
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Wang, X. R.; Tan, G. Q.; Bai, Y.; Wu, F.; Wu, C. Multi-electron reaction materials for high-energy-density secondary batteries: Current status and prospective. Electrochem. Energy Rev. 2021, 4, 35–66.
Huo, X. G.; Zhong, J. J.; Yang, Z.; Feng, J. M.; Li, J. L.; Kang, F. Y. In situ preparation of MXenes in ambient-temperature organic ionic liquid aluminum batteries with ultrastable cycle performance. ACS Appl. Mater. Interfaces 2021, 13, 55112–55122.
Jayaprakash, N.; Das, S. K.; Archer, L. A. The rechargeable aluminum-ion battery. Chem. Commun. 2011, 47, 12610–12612.
Zu, C. X.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624.
Elia, G. A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R. Y.; Knipping, E.; Peters, W.; Drillet, J. F.; Passerini, S.; Hahn, R. An overview and future perspectives of aluminum batteries. Adv. Mater. 2016, 28, 7564–7579.
Lin, M. C.; Gong, M.; Lu, B. G.; Wu, Y. P.; Wang, D. Y.; Guan, M. Y.; Angell, M.; Chen, C. X.; Yang, J.; Hwang, B. J. et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328.
Yu, X. Z.; Wang, B.; Gong, D. C.; Xu, Z.; Lu, B. G. Graphene nanoribbons on highly porous 3D graphene for high-capacity and ultrastable Al-ion batteries. Adv. Mater. 2017, 29, 1604118.
Sun, H. B.; Wang, W.; Yu, Z. J.; Yuan, Y.; Wang, S.; Jiao, S. Q. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 2015, 51, 11892–11895.
Jiao, H. D.; Wang, C.; Tu, J. G.; Tian, D. H.; Jiao, S. Q. A rechargeable Al-ion battery: Al/molten AlCl3-urea/graphite. Chem. Commun. 2017, 53, 2331–2334.
Rani, J. V.; Kanakaiah, V.; Dadmal, T.; Rao, M. S.; Bhavanarushi, S. Fluorinated natural graphite cathode for rechargeable ionic liquid based aluminum-ion battery. J. Electrochem. Soc. 2013, 160, A1781–A1784.
Jiao, S. Q.; Lei, H. P.; Tu, J. G.; Zhu, J.; Wang, J. X.; Mao, X. H. An industrialized prototype of the rechargeable Al/AlCl3−[EMIm]Cl/graphite battery and recycling of the graphitic cathode into graphene. Carbon 2016, 109, 276–281.
Tu, J. G.; Wang, J. X.; Li, S. J.; Song, W. L.; Wang, M. Y.; Zhu, H. M.; Jiao, S. Q. High-efficiency transformation of amorphous carbon into graphite nanoflakes for stable aluminum-ion battery cathodes. Nanoscale 2019, 11, 12537–12546.
Wang, P.; Chen, H. S.; Li, N.; Zhang, X. Y.; Jiao, S. Q.; Song, W. L.; Fang, D. N. Dense graphene papers: Toward stable and recoverable Al-ion battery cathodes with high volumetric and areal energy and power density. Energy Storage Mater. 2018, 13, 103–111.
Wang, H. L.; Bai, Y.; Chen, S.; Luo, X. Y.; Wu, C.; Wu, F.; Lu, J.; Amine, K. Binder-free V2O5 cathode for greener rechargeable aluminum battery. ACS Appl. Mater. Interfaces 2015, 7, 80–84.
Wang, H. L.; Bi, X. X.; Bai, Y.; Wu, C.; Gu, S. C.; Chen, S.; Wu, F.; Amine, K.; Lu, J. Open-structured V2O5·nH2O nanoflakes as highly reversible cathode material for monovalent and multivalent intercalation batteries. Adv. Energy Mater. 2017, 7, 1602720.
Wang, H. L.; Gu, S. C.; Bai, Y.; Chen, S.; Wu, F.; Wu, C. High-voltage and noncorrosive ionic liquid electrolyte used in rechargeable aluminum battery. ACS Appl. Mater. Interfaces 2016, 8, 27444–27448.
Liu, S.; Hu, J. J.; Yan, N. F.; Pan, G. L.; Li, G. R.; Gao, X. P. Aluminum storage behavior of anatase TiO2 nanotube arrays in aqueous solution for aluminum ion batteries. Energy Environ. Sci. 2012, 5, 9743–9746.
Liu, Y. Y.; Sang, S. B.; Wu, Q. M.; Lu, Z. G.; Liu, K. Y.; Liu, H. T. The electrochemical behavior of Cl assisted Al3+ insertion into titanium dioxide nanotube arrays in aqueous solution for aluminum ion batteries. Electrochim. Acta 2014, 143, 340–346.
Kumar, S.; Satish, R.; Verma, V.; Ren, H.; Kidkhunthod, P.; Manalastas, W.; Srinivasan, M. Investigating FeVO4 as a cathode material for aqueous aluminum-ion battery. J. Power Sources 2019, 426, 151–161.
Xiao, X.; Wang, M. Y.; Tu, J. G.; Luo, Y. W.; Jiao, S. Q. Metal-organic framework-derived Co3O4@MWCNTs polyhedron as cathode material for a high-performance aluminum-ion battery. ACS Sustainable Chem. Eng. 2019, 7, 16200–16208.
Gao, T.; Li, X. G.; Wang, X. W.; Hu, J. K.; Han, F. D.; Fan, X. L.; Suo, L. M.; Pearse, A. J.; Lee, S. B.; Rubloff, G. W. et al. A rechargeable Al/S battery with an ionic-liquid electrolyte. Angew. Chem., Int. Ed. 2016, 55, 9898–9901.
Wang, S.; Jiao, S. Q.; Wang, J. X.; Chen, H. S.; Tian, D. H.; Lei, H. P.; Fang, D. N. High-performance aluminum-ion battery with CuS@C microsphere composite cathode. ACS Nano 2017, 11, 469–477.
Zhao, Z. C.; Hu, Z. Q.; Li, Q.; Li, H. S.; Zhang, X.; Zhuang, Y. D.; Wang, F.; Yu, G. H. Designing two-dimensional WS2 layered cathode for high-performance aluminum-ion batteries: From micro-assemblies to insertion mechanism. Nano Today 2020, 32, 100870.
Wang, S.; Yu, Z. J.; Tu, J. G.; Wang, J. X.; Tian, D. H.; Liu, Y. J.; Jiao, S. Q. A novel aluminum-ion battery: Al/AlCl3−[EMIm]Cl/Ni3S2@graphene. Adv. Energy Mater. 2016, 6, 1600137.
Geng, L. X.; Lv, G. C.; Xing, X. B.; Guo, J. C. Cheminform abstract: Reversible electrochemical intercalation of aluminum in Mo6S8. Cheminform 2015, 46, 4926–4929.
Yu, Z. J.; Kang, Z. P.; Hu, Z. Q.; Lu, J. H.; Zhou, Z. G.; Jiao, S. Q. Hexagonal NiS nanobelts as advanced cathode materials for rechargeable Al-ion batteries. Chem. Commun. 2016, 52, 10427–10430.
Hu, Y. X.; Luo, B.; Ye, D. L.; Zhu, X. B.; Lyu, M. Q.; Wang, L. Z. An innovative freeze-dried reduced graphene oxide supported SnS2 cathode active material for aluminum-ion batteries. Adv. Mater. 2017, 29, 1606132.
Tan, B.; Han, S. H.; Luo, W. B.; Chao, Z. S.; Fan, J. C.; Wang, M. Y. Synthesis of RGO-supported layered MoS2 with enhanced electrochemical performance for aluminum ion batteries. J. Alloys Compd. 2020, 841, 155732.
Li, Z. Y.; Wang, X. X.; Li, X. X.; Zhang, W. M. Reduced graphene oxide (rGO) coated porous nanosphere TiO2@Se composite as cathode material for high-performance reversible Al−Se batteries. Chem. Eng. J. 2020, 400, 126000.
Li, Z. Y.; Wang, X. X.; Zhang, W. M.; Yang, S. P. Two-dimensional Ti3C2@CTAB-Se (MXene) composite cathode material for high-performance rechargeable aluminum batteries. Chem. Eng. J. 2020, 398, 125679.
Cai, T. H.; Zhao, L. M.; Hu, H. Y.; Li, T. G.; Li, X. C.; Guo, S.; Li, Y. P.; Xue, Q. Z.; Xing, W.; Yan, Z. F. et al. Stable CoSe2/carbon nanodice@reduced graphene oxide composites for high-performance rechargeable aluminum-ion batteries. Energy Environ. Sci. 2018, 11, 2341–2347.
Guan, W.; Wang, L. J.; Lei, H. P.; Tu, J. G.; Jiao, S. Q. Sb2Se3 nanorods with N-doped reduced graphene oxide hybrids as high-capacity positive electrode materials for rechargeable aluminum batteries. Nanoscale 2019, 11, 16437–16444.
Jiang, J. L.; Li, H.; Fu, T.; Hwang, B. J.; Li, X.; Zhao, J. B. One-dimensional Cu2−xSe nanorods as the cathode material for high-performance aluminum-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 17942–17949.
Zhao, Z. C.; Hu, Z. Q.; Liang, H. Y.; Li, S. D.; Wang, H. T.; Gao, F.; Sang, X. C.; Li, H. S. Nanosized MoSe2@carbon matrix: A stable host material for the highly reversible storage of potassium and aluminum ions. ACS Appl. Mater. Interfaces 2019, 11, 44333–44341.
Huo, X. G.; Liu, J.; Li, J. L.; Zhang, B.; Zhang, Y.; Yu, Y.; Kang, F. Y. Hexagonal composite CuSe@C as a positive electrode for high-performance aluminum batteries. ACS Appl. Energy Mater. 2020, 3, 11445–11455.
Lei, H. P.; Wang, M. Y.; Tu, J. G.; Jiao, S. Q. Single-crystal and hierarchical VSe2 as an aluminum-ion battery cathode. Sustainable Energy Fuels 2019, 3, 2717–2724.
Zhang, Y.; Zhang, B.; Li, J. L.; Liu, J.; Huo, X. G.; Kang, F. Y. SnSe nano-particles as advanced positive electrode materials for rechargeable aluminum-ion batteries. Chem. Eng. J. 2021, 403, 126377.
Li, G. Y.; Kou, M. Y.; Tu, J. G.; Luo, Y. W.; Wang, M. Y.; Jiao, S. Q. Coordination interaction boosts energy storage in rechargeable Al battery with a positive electrode material of CuSe. Chem. Eng. J. 2021, 421, 127792.
Li, J. J.; Liu, W. M.; Yu, Z. Z.; Deng, J. Q.; Zhong, S. K.; Xiao, Q.; Chen, F. M.; Yan, D. L. N-doped C@ZnSe as a low cost positive electrode for aluminum-ion batteries: Better electrochemical performance with high voltage platform of ∼ 1.8 V and new reaction mechanism. Electrochim. Acta 2021, 370, 137790.
Lv, W. R.; Wu, G. H.; Li, X. X.; Li, J. L.; Li, Z. Y. Two-dimensional V2C@Se (MXene) composite cathode material for high-performance rechargeable aluminum batteries. Energy Storage Mater. 2022, 46, 138–146.
Zhang, X. F.; Zhang, G. H.; Wang, S.; Li, S. J.; Jiao, S. Q. Porous CuO microsphere architectures as high-performance cathode materials for aluminum-ion batteries. J. Mater. Chem. A 2018, 6, 3084–3090.
Wang, S.; Tu, J. G.; Xiao, J. S.; Zhu, J.; Jiao, S. Q. 3D skeleton nanostructured Ni3S2/Ni foam@RGO composite anode for high-performance dual-ion battery. J. Energy Chem. 2019, 28, 144–150.
Zhang, X. F.; Wang, S.; Tu, J. G.; Zhang, G. H.; Li, S. J.; Tian, D. H.; Jiao, S. Q. Flower-like vanadium suflide/reduced graphene oxide composite: An energy storage material for aluminum-ion batteries. ChemSusChem 2018, 11, 709–715.
Xing, W.; Li, X. C.; Cai, T. H.; Zhang, Y.; Bai, P.; Xu, J.; Hu, H.; Wu, M. B.; Xue, Q. Z.; Zhao, Y. et al. Layered double hydroxides derived NiCo-sulfide as a cathode material for aluminum ion batteries. Electrochim. Acta 2020, 344, 136174.
Wu, S. C.; Ai, Y. F.; Chen, Y. Z.; Wang, K. Y.; Yang, T. Y.; Liao, H. J.; Su, T. Y.; Tang, S. Y.; Chen, C. W.; Wu, D. C. et al. High-performance rechargeable aluminum-selenium battery with a new deep eutectic solvent electrolyte: Thiourea-AlCl3. ACS Appl. Mater. Interfaces 2020, 12, 27064–27073.
Kao, Y. T.; Patil, S. B.; An, C. Y.; Huang, S. K.; Lin, J. C.; Lee, T. S.; Lee, Y. C.; Chou, H. L.; Chen, C. W.; Chang, Y. J. et al. A quinone-based electrode for high-performance rechargeable aluminum-ion batteries with a low-cost AlCl3/Urea ionic liquid electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 25853–25860.
Li, G. Y.; Tu, J. G.; Wang, M. Y.; Jiao, S. Q. Cu3P as a novel cathode material for rechargeable aluminum-ion batteries. J. Mater. Chem. A 2019, 7, 8368–8375.
Zhang, B.; Zhang, Y.; Li, J. L.; Liu, J.; Huo, X. G.; Kang, F. Y. In situ growth of metal-organic framework-derived CoTe2 nanoparticles@nitrogen-doped porous carbon polyhedral composites as novel cathodes for rechargeable aluminum-ion batteries. J. Mater. Chem. A 2020, 8, 5535–5545.
Yu, Z. J.; Jiao, S. Q.; Tu, J. G.; Luo, Y. W.; Song, W. L.; Jiao, H. D.; Wang, M. Y.; Chen, H. S.; Fang, D. N. Rechargeable nickel telluride/aluminum batteries with high capacity and enhanced cycling performance. ACS Nano 2020, 14, 3469–3476.
Li, Z. Y.; Liu, J.; Huo, X. G.; Li, J. L.; Kang, F. Y. Novel one-dimensional hollow carbon nanotubes/selenium composite for highperformance Al-Se batteries. ACS Appl. Mater. Interfaces 2019, 11, 45709–45716.
Zhuang, R. Y.; Miao, G.; Huang, Z. L.; Zhang, Q. Q.; Wu, J. C.; Yang, J. H. Non-stoichiometric CoS1.097 nanoparticles prepared from CoAl-layered double hydroxide and MOF template as cathode materials for aluminum-ion batteries. J. Energy Chem. 2021, 54, 639–643.
Zhuang, R. Y.; Huang, Z. L.; Wang, S. X.; Qiao, J.; Wu, J. C.; Yang, J. H. Binder-free cobalt sulfide@carbon nanofibers composite films as cathode for rechargeable aluminum-ion batteries. Chem. Eng. J. 2021, 409, 128235.
Huo, X. G.; Wang, X. X.; Li, Z. Y.; Liu, J.; Li, J. L. Two-dimensional composite of D-Ti3C2Tx@S@TiO2 (MXene) as the cathode material for aluminum-ion batteries. Nanoscale 2020, 12, 3387–3399.
Lei, H. P.; Jiao, S. Q.; Tu, J. G.; Song, W. L.; Zhang, X. F.; Wang, M. Y.; Li, S. J.; Chen, H. S.; Fang, D. N. Modified separators for rechargeable high-capacity selenium-aluminium batteries. Chem. Eng. J. 2020, 385, 123452.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52102233) and Nature Science Foundation of Hebei Province (No. E2021201006).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, X., Ma, M., Lv, W. et al. CMK-3 modified separator for ultra-high stability performance Cu1.8Se aluminum batteries. Nano Res. 15, 8136–8145 (2022). https://doi.org/10.1007/s12274-022-4517-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4517-x