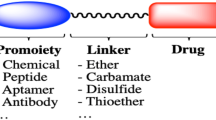
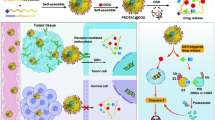
Abstract
The cathepsin B-responsive prodrugs are promising strategies to reduce the serious adverse effects of anticancer drugs by improving the cancer selectivity that can be specifically activated by overexpressed cathepsin B in targeted cancer cells. However, clinical translation of such therapeutic approaches has been restricted by low antitumor efficacy that is mainly attributable to undesirable pharmacokinetic profiles and inefficient tumor-targeting of cathepsin B-responsive prodrugs, due to their small-molecule structure. In recent decades, many researchers have widely investigated the drug delivery system (DDS) to improve the in vivo pharmacokinetic profiles and tumor-targeting efficiency of cathepsin B-responsive prodrugs via the application of polymers, dendrimers, antibodies, lipids, and inorganic nanoparticles as drug carriers. In addition, the potential therapeutic efficacy of DDS for cathepsin B-responsive prodrugs is demonstrated in multiple studies and combinatorial treatment with typical therapeutic modalities can effectively overcome the challenges of tumor heterogeneity and multidrug resistance. In this review, recent advances and progress of new DDS for cathepsin B-responsive prodrugs are outlined, and their clinical trials are discussed. Besides, potential challenges and the outlooks for clinical translation of cathepsin B-responsive prodrugs are highlighted.

Similar content being viewed by others
References
Pathania, D.; Millard, M.; Neamati, N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev. 2009, 61, 1250–1275.
Tacar, O.; Sriamornsak, P.; Dass, C. R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170.
Petru, E.; Schmähl, D. Cytotoxic chemotherapy-induced second primary neoplasms: Clinical aspects. Neoplasma 1991, 38, 147–155.
Rautio, J.; Meanwell, N. A.; Di, L.; Hageman, M. J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 2018, 17, 559–587.
Zeng, Z. L.; Zhang, C.; Li, J. C.; Cui, D.; Jiang, Y. Y.; Pu, K. Y. Activatable polymer nanoenzymes for photodynamic immunometabolic cancer therapy. Adv. Mater. 2021, 33, 2007247.
Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270.
Zhang, C.; Pu, K. Y. Molecular and nanoengineering approaches towards activatable cancer immunotherapy. Chem. Soc. Rev. 2020, 49, 4234–4253.
Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H. Y.; Shim, M. K.; Lee, S.; Ko, Y. J.; Kim, M. J.; Park, J. H. et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494.
Kim, J.; Shim, M. K.; Cho, Y. J.; Jeon, S.; Moon, Y.; Choi, J.; Kim, J.; Lee, J.; Lee, J. W.; Kim, K. The safe and effective intraperitoneal chemotherapy with cathepsin B-specific doxorubicin prodrug nanoparticles in ovarian cancer with peritoneal carcinomatosis. Biomaterials 2021, 279, 121189.
Sun, I. C.; Yoon, H. Y.; Lim, D. K.; Kim, K. Recent trends in in situ enzyme-activatable prodrugs for targeted cancer therapy. Bioconjugate Chem. 2020, 31, 1012–1024.
Shim, M. K.; Yoon, H. Y.; Lee, S.; Jo, M. K.; Park, J.; Kim, J. H.; Jeong, S. Y.; Kwon, I. C.; Kim, K. Caspase-3/-7-specific metabolic precursor for bioorthogonal tracking of tumor apoptosis. Sci. Rep. 2017, 7, 16635.
Shim, M. K.; Yang, S.; Sun, I. C.; Kim, K. Tumor-activated carrier-free prodrug nanoparticles for targeted cancer immunotherapy: Preclinical evidence for safe and effective drug delivery. Adv. Drug Deliv. Rev. 2022, 183, 114177.
Ruan, H.; Hao, S. S.; Young, P.; Zhang, H. T. Targeting cathepsin B for cancer therapies. Horiz. Cancer Res. 2015, 56, 23–40.
Zhang, C.; Zeng, Z. L.; Cui, D.; He, S. S.; Jiang, Y. Y.; Li, J. C.; Huang, J. G.; Pu, K. Y. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat. Commun. 2021, 12, 2934.
Shim, M. K.; Yoon, H. Y.; Ryu, J. H.; Koo, H.; Lee, S.; Park, J. H.; Kim, J. H.; Lee, S.; Pomper, M. G.; Kwon, I. C. et al. Cathepsin B-specific metabolic precursor for in vivo tumor-specific fluorescence imaging. Angew. Chem., Int. Ed. 2016, 55, 14698–14703.
Reinheckel, T.; Deussing, J.; Roth, W.; Peters, C. Towards specific functions of lysosomal cysteine peptidases: Phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 2001, 382, 735–742.
Aggarwal, N.; Sloane, B. F. Cathepsin B: Multiple roles in cancer. Proteomics Clin. Appl. 2014, 8, 427–437.
Zhong, Y. J.; Shao, L. H.; Li, Y. Cathepsin B-cleavable doxorubicin prodrugs for targeted cancer therapy (Review). Int. J. Oncol. 2013, 42, 373–383.
Shim, M. K.; Park, J.; Yoon, H. Y.; Lee, S.; Um, W.; Kim, J. H.; Kang, S. W.; Seo, J. W.; Hyun, S. W.; Park, J. H. et al. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J. Control. Release 2019, 294, 376–389.
Moon, Y.; Shim, M. K.; Choi, J.; Yang, S.; Kim, J.; Yun, W. S.; Cho, H.; Park, J. Y.; Kim, Y.; Seong, J. K. et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics 2022, 12, 1999–2014.
Cho, H.; Shim, M. K.; Yang, S.; Song, S.; Moon, Y.; Kim, J.; Byun, Y.; Ahn, C. H.; Kim, K. Cathepsin B-overexpressed tumor cell activatable albumin-binding doxorubicin prodrug for cancer-targeted therapy. Pharmaceutics 2021, 14, 83.
Tang, L.; Duan, R.; Zhong, Y. J.; Firestone, R. A.; Hong, Y. P.; Li, J. G.; Xin, Y. C.; Wu, H. L.; Li, Y. Synthesis, identification and in vivo studies of tumor-targeting agent peptide doxorubicin (PDOX) to treat peritoneal carcinomatosis of gastric cancer with similar efficacy but reduced toxicity. Mol. Cancer 2014, 13, 44.
de Groot, F. M. H.; Broxterman, H. J.; Adams, H. P. H. M.; van Vliet, A.; Tesser, G. I.; Elderkamp, Y. W.; Schraa, A. J.; Kok, R. J.; Molema, G.; Pinedo, H. M. et al. Design, synthesis, and biological evaluation of a dual tumor-specific motive containing integrin-targeted plasmin-cleavable doxorubicin prodrug. Mol. Cancer Ther. 2002, 1, 901–911.
Dubowchik, G. M.; Firestone, R. A.; Padilla, L.; Willner, D.; Hofstead, S. J.; Mosure, K.; Knipe, J. O.; Lasch, S. J.; Trail, P. A. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: Model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjugate Chem. 2002, 13, 855–869.
Yang, B.; Gao, J.; Pei, Q.; Xu, H. X.; Yu, H. J. Engineering prodrug nanomedicine for cancer immunotherapy. Adv. Sci. 2020, 7, 2002365.
Bertrand, N.; Wu, J.; Xu, X. Y.; Kamaly, N.; Farokhzad, O. C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25.
Lim, S.; Park, J.; Shim, M. K.; Um, W.; Yoon, H. Y.; Ryu, J. H.; Lim, D. K.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906–7923.
Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323.
Li, Y. N.; Mei, T.; Han, S. P.; Han, T.; Sun, Y. B.; Zhang, H.; An, F. F. Cathepsin B-responsive nanodrug delivery systems for precise diagnosis and targeted therapy of malignant tumors. Chin. Chem. Lett. 2020, 31, 3027–3040.
Delplace, V.; Couvreur, P.; Nicolas, J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym. Chem. 2014, 5, 1529–1544.
Dragojevic, S.; Ryu, J. S.; Raucher, D. Polymer-based prodrugs: Improving tumor targeting and the solubility of small molecule drugs in cancer therapy. Molecules 2015, 20, 21750–21769.
Duncan, R.; Kopečková-Rejmanová, P.; Strohalm, J.; Hume, I.; Cable, H. C.; Pohl, J.; Lloyd, J. B.; Kopeček, J. Anticancer agents coupled to N-(2-hydroxypropyl) methacrylamide copolymers. I. Evaluation of daunomycin and puromycin conjugates in vitro. Br. J. Cancer 1987, 55, 165–174.
Kopeček, J.; Baẑilová, H. Poly[N-(2-hydroxypropyl) methacrylamide]—I. Radical polymerization and copolymerization. Eur. Polym. J. 1973, 9, 7–14.
Yang, J. Y.; Kopeček, J. The light at the end of the tunnel—Second generation HPMA conjugates for cancer treatment. Curr. Opin. Colloid Interface Sci. 2017, 31, 30–42.
Duncan, R. Development of HPMA copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv. Drug Deliv. Rev. 2009, 61, 1131–1148.
Seymour, L. W.; Ferry, D. R.; Kerr, D. J.; Rea, D.; Whitlock, M.; Poyner, R.; Boivin, C.; Hesslewood, S.; Twelves, C.; Blackie, R. et al. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int. J. Oncol. 2009, 34, 1629–1636.
Julyan, P. J.; Seymour, L. W.; Ferry, D. R.; Daryani, S.; Boivin, C. M.; Doran, J.; David, M.; Anderson, D.; Christodoulou, C.; Young, A. M. et al. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. J. Control. Release 1999, 57, 281–290.
Terwogt, J. M. M.; ten Bokkel Huinink, W. W.; Schellens, J. H. M.; Schot, M.; Mandjes, I. A. M.; Zurlo, M. G.; Rocchetti, M.; Rosing, H.; Koopman, F. J.; Beijnen, J. H. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anti-Cancer Drugs 2001, 12, 315–323.
Schoemaker, N. E.; van Kesteren, C.; Rosing, H.; Jansen, S.; Swart, M.; Lieverst, J.; Fraier, D.; Breda, M.; Pellizzoni, C.; Spinelli, R. et al. A phase I and pharmacokinetic study of MAG-CPT, a water-soluble polymer conjugate of camptothecin. Br. J. Cancer 2002, 87, 608–614.
Dvořák, M.; Kopečková, P.; Kopeček, J. High-molecular weight HPMA copolymer-adriamycin conjugates. J. Control. Release 1999, 60, 321–332.
Shiah, J. G.; Dvořák, M.; Kopečková, P.; Sun, Y.; Peterson, C. M.; Kopeček, J. Biodistribution and antitumour efficacy of long-circulating N-(2-hydroxypropyl)methacrylamide copolymer-doxorubicin conjugates in nude mice. Eur. J. Cancer 2001, 37, 131–139.
Pan, H. Z.; Sima, M.; Miller, S. C.; Kopečková, P.; Yang, J. Y.; Kopeček, J. Efficiency of high molecular weight backbone degradable HPMA copolymer-prostaglandin E1 conjugate in promotion of bone formation in ovariectomized rats. Biomaterials 2013, 34, 6528–6538.
Zhang, R.; Luo, K.; Yang, J. Y.; Sima, M.; Sun, Y. E.; Janát-Amsbury, M. M.; Kopeček, J. Synthesis and evaluation of a backbone biodegradable multiblock HPMA copolymer nanocarrier for the systemic delivery of paclitaxel. J. Control. Release 2013, 166, 66–74.
Yang, J. Y.; Luo, K.; Pan, H. Z.; Kopečková, P.; Kopeček, J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. React. Funct. Polym. 2011, 71, 294–302.
Pan, H. Z.; Yang, J. Y.; Kopečková, P.; Kopeček, J. Backbone degradable multiblock N-(2-hydroxypropyl)methacrylamide copolymer conjugates via reversible addition-fragmentation chain transfer polymerization and thiol—ene coupling reaction. Biomacromolecules 2011, 12, 247–252.
Pan, H. Z.; Sima, M.; Yang, J. Y.; Kopeček, J. Synthesis of long-circulating, backbone degradable HPMA copolymer-doxorubicin conjugates and evaluation of molecular-weight-dependent antitumor efficacy. Macromol. Biosci. 2013, 13, 155–160.
Sponchioni, M.; Morosi, L.; Lupi, M.; Palmiero, U. C. Poly(HPMA)-based copolymers with biodegradable side chains able to self assemble into nanoparticles. RSC Adv. 2017, 7, 50981–50992.
Yang, Y.; Pan, D. Y.; Luo, K.; Li, L.; Gu, Z. W. Biodegradable and amphiphilic block copolymer-doxorubicin conjugate as polymeric nanoscale drug delivery vehicle for breast cancer therapy. Biomaterials 2013, 34, 8430–8443.
Dai, Y.; Ma, X. L.; Zhang, Y. H.; Chen, K.; Tang, J. Z.; Gong, Q. Y.; Luo, K. A biocompatible and cathepsin B sensitive nanoscale system of dendritic polyHPMA-gemcitabine prodrug enhances antitumor activity markedly. Biomater. Sci. 2018, 6, 2976–2986.
Cai, H.; Dai, X. H.; Wang, X. M.; Tan, P.; Gu, L.; Luo, Q.; Zheng, X. L.; Li, Z. Q.; Zhu, H. Y.; Zhang, H. et al. A nanostrategy for efficient imaging-guided antitumor therapy through a stimuli-responsive branched polymeric prodrug. Adv. Sci. 2020, 7, 1903243.
Yang, J. Y.; Zhang, R.; Pan, H. Z.; Li, Y. L.; Fang, Y. X.; Zhang, L. B.; Kopeček, J. Backbone degradable N-(2-hydroxypropyl)methacrylamide copolymer conjugates with gemcitabine and paclitaxel: Impact of molecular weight on activity toward human ovarian carcinoma xenografts. Mol. Pharmaceutics 2017, 14, 1384–1394.
Zhang, R.; Yang, J. Y.; Sima, M.; Zhou, Y.; Kopeček, J. Sequential combination therapy of ovarian cancer with degradable N-(2-hydroxypropyl)methacrylamide copolymer paclitaxel and gemcitabine conjugates. Proc. Natl. Acad. Sci. USA 2014, 111, 12181–12186.
Duangjai, A.; Luo, K.; Zhou, Y.; Yang, J. Y.; Kopeček, J. Combination cytotoxicity of backbone degradable HPMA copolymer gemcitabine and platinum conjugates toward human ovarian carcinoma cells. Eur. J. Pharm. Biopharm. 2014, 87, 187–196.
Zhou, Y.; Yang, J. Y.; Zhang, R.; Kopeček, J. Combination therapy of prostate cancer with HPMA copolymer conjugates containing PI3K/mTOR inhibitor and docetaxel. Eur. J. Pharm. Biopharm. 2015, 89, 107–115.
Zhou, Y.; Yang, J. Y.; Rhim, J. S.; Kopeček, J. HPMA copolymer-based combination therapy toxic to both prostate cancer stem/progenitor cells and differentiated cells induces durable antitumor effects. J. Control. Release 2013, 172, 946–953.
Maloth, K. N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vellamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic therapy—A non-invasive treatment modality for precancerous lesions. J. Lasers Med. Sci. 2016, 7, 30–36.
Dalpiaz, A.; Paganetto, G.; Botti, G.; Pavan, B. Cancer stem cells and nanomedicine: New opportunities to combat multidrug resistance. Drug Discov. Today 2020, 25, 1651–1667.
Zhen, S. J.; Yi, X. Q.; Zhao, Z. J.; Lou, X. D.; Xia, F.; Tang, B. Z. Drug delivery micelles with efficient near-infrared photosensitizer for combined image-guided photodynamic therapy and chemotherapy of drug-resistant cancer. Biomaterials 2019, 218, 119330.
Krinick, N. L.; Sun, Y.; Joyner, D.; Spikes, J. D.; Straight, R. C.; Kopeček, J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J. Biomater. Sci., Polym. Ed. 1994, 5, 303–324.
Peterson, C. M.; Lu, J. M.; Sun, Y.; Peterson, C. A.; Shiah, J. G.; Straight, R. C.; Kopeček, J. Combination chemotherapy and photodynamic therapy with N-(2-hydroxypropyl)methacrylamide copolymer-bound anticancer drugs inhibit human ovarian carcinoma heterotransplanted in nude mice. Cancer Res. 1996, 56, 3980–3985.
Shiah, J. G.; Sun, Y.; Kopečková, P.; Peterson, C. M.; Straight, R. C.; Kopeček, J. Combination chemotherapy and photodynamic therapy of targetable N-(2-hydroxypropyl)methacrylamide copolymer-doxorubicin/mesochlorin e6-OV-TL 16 antibody immunoconjugates. J. Control. Release 2001, 74, 249–253.
Scomparin, A.; Florindo, H. F.; Tiram, G.; Ferguson, E. L.; Satchi-Fainaro, R. Two-step polymer-and liposome-enzyme prodrug therapies for cancer: PDEPT and PELT concepts and future perspectives. Adv. Drug Deliv. Rev. 2017, 118, 52–64.
Haag, R.; Kratz, F. Polymer therapeutics: Concepts and applications. Angew. Chem., Int. Ed. 2006, 45, 1198–1215.
Satchi, R.; Connors, T. A.; Duncan, R. PDEPT: Polymer-directed enzyme prodrug therapy. Br. J. Cancer 2001, 35, 1070–1076.
Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008, 15, 3–12.
Krysko, D. V.; Garg, A. D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875.
Bilusic, M.; Gulley, J. L. Editorial: Local immunotherapy: A way to convert tumors from “cold” to “hot”. JNCI:J. Natl. Cancer Inst. 2017, 109, djx132.
Li, L.; Li, Y. C.; Yang, C. H.; Radford, D. C.; Wang, J. W.; Janát-Amsbury, M.; Kopeček, J.; Yang, J. Y. Inhibition of immunosuppressive tumors by polymer-assisted inductions of immunogenic cell death and multivalent PD-L1 crosslinking. Adv. Funct. Mater. 2020, 30, 1908961.
Li, L.; Wang, J. W.; Radford, D. C.; Kopeček, J.; Yang, J. Y. Combination treatment with immunogenic and anti-PD-L1 polymer-drug conjugates of advanced tumors in a transgenic MMTV-PyMT mouse model of breast cancer. J. Control. Release 2021, 332, 652–659.
Liang, L.; Lin, S. W.; Dai, W. B.; Lu, J. K.; Yang, T. Y.; Xiang, Y.; Zhang, Y.; Li, R. T.; Zhang, Q. Novel cathepsin B-sensitive paclitaxel conjugate: Higher water solubility, better efficacy and lower toxicity. J. Control. Release 2012, 160, 618–629.
Lin, J. Y.; Pan, Z.; Song, L.; Zhang, Y. M.; Li, Y.; Hou, Z. Q.; Lin, C. J. Design and in vitro evaluation of self-assembled indometacin prodrug nanoparticles for sustained/controlled release and reduced normal cell toxicity. Appl. Surf. Sci. 2017, 425, 674–681.
Zhang, X.; Tang, K. Y.; Wang, H.; Liu, Y. Q.; Bao, B.; Fang, Y. F.; Zhang, X. W.; Lu, W. Design, synthesis, and biological evaluation of new cathepsin B-sensitive camptothecin nanoparticles equipped with a novel multifuctional linker. Bioconjugate Chem. 2016, 27, 1267–1275.
Lu, S.; Lei, X.; Ren, H.; Zheng, S. Y.; Qiang, J.; Zhang, Z. J.; Chen, Y. H.; Wei, T. W.; Wang, F.; Chen, X. Q. PEGylated dimeric BODIPY photosensitizers as nanocarriers for combined chemotherapy and cathepsin B-activated photodynamic therapy in 3D tumor spheroids. ACS Appl. Bio Mater. 2020, 3, 3835–3845.
Chapman, A. P. PEGylated antibodies and antibody fragments for improved therapy: A review. Adv. Drug Deliv. Rev. 2002, 54, 531–545.
Veronese, F. M. PEGylated Protein Drugs: Basic Science and Clinical Applications; Birkhäuser: Basel, 2009.
Dai, C. Y.; Fu, Y.; Li, B.; Wang, Y. G.; Zhang, X.; Wang, J. C.; Zhang, Q. Linkage with cathepsin B-sensitive dipeptide promotes the in vitro and in vivo anticancer activity of PEGylated tumor necrosis factor-alpha (TNF-α) against murine fibrosarcoma. Sci. China Life Sci. 2011, 54, 128–138.
Dai, C. Y.; Fu, Y.; Chen, S. C.; Li, B.; Yao, B.; Liu, W. H.; Zhu, L. Q.; Chen, N.; Chen, J.; Zhang, Q. Preparation and evaluation of a new releasable PEGylated tumor necrosis factor-α (TNF-α) conjugate for therapeutic application. Sci. China Life Sci. 2013, 56, 51–58.
Tan, P.; Cai, H.; Wei, Q.; Tang, X. D.; Zhang, Q. F.; Kopytynski, M.; Yang, J. X.; Yi, Y.; Zhang, H.; Gong, Q. Y. et al. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials 2021, 277, 121061.
Luo, Q.; Lin, L.; Huang, Q.; Duan, Z.; Gu, L.; Zhang, H.; Gu, Z.; Gong, Q.; Luo, K. Dual stimuli-responsive dendronized prodrug derived from poly(oligo-(ethylene glycol) methacrylate)-based copolymers for enhanced anti-cancer therapeutic effect. Acta Biomater. 2022, 143, 320–332.
Herceg, V.; Bouilloux, J.; Janikowska, K.; Allámann, E.; Lange, N. Cathepsin B-cleavable cyclopeptidic chemotherapeutic prodrugs. Molecules 2020, 25, 4285.
Dai, J.; Hu, J. J.; Dong, X. Q.; Chen, B.; Dong, X. Y.; Liu, R.; Xia, F.; Lou, X. D. Deep downregulation of PD-L1 by caged peptide-conjugated AIEgen/miR-140 nanoparticles for enhanced immunotherapy. Angew. Chem., Int. Ed., in press, https://doi.org/10.1002/anie.202117798.
Coessens, V.; Schacht, E. H.; Domurado, D. Synthesis and in vitro stability of macromolecular prodrugs of norfloxacin. J. Control. Release 1997, 47, 283–291.
Nichifor, M.; Schacht, E. H.; Seymour, L. W. Polymeric prodrugs of 5-fluorouracil. J. Control. Release 1997, 48, 165–178.
Harada, M.; Sakakibara, H.; Yano, T.; Suzuki, T.; Okuno, S. Determinants for the drug release from T-0128, camptothecin analogue-carboxymethyl dextran conjugate. J. Control. Release 2000, 69, 399–412.
Ouchi, T.; Tada, M.; Matsumoto, M.; Ohya, Y.; Hasegawa, K.; Arai, Y.; Kadowaki, K.; Akao, S.; Matsumoto, T.; Suzuki, S. et al. Design of macromolecular prodrug of 5-fluorouracil using N-acetylpolygalactosamine as a targeting carrier to hepatoma. React. Funct. Polym. 1998, 37, 235–244.
Ouchi, T.; Tada, M.; Matsumoto, M.; Ohya, Y.; Hasegawa, K.; Arai, Y.; Kadowaki, K.; Akao, S.; Matsumoto, T.; Suzuki, S. et al. Design of lysosomotropic macromolecular prodrug of doxorubicin using N-acetyl-α-1, 4-polygalactosamine as a targeting carrier to hepatoma tissue. J. Bioact. Compat. Polym. 1998, 13, 257–269.
Pan, X.; Chen, J. R.; Yang, M. D.; Wu, J.; He, G. H.; Yin, Y. H.; He, M.; Xu, W. J.; Xu, P. H.; Cai, W. Q. et al. Enzyme/pH dual-responsive polymer prodrug nanoparticles based on 10-hydroxycamptothecin-carboxymethylchitosan for enhanced drug stability and anticancer efficacy. Eur. Polym. J. 2019, 117, 372–381.
Zhang, X. D.; He, F.; Xiang, K. Q.; Zhang, J. J.; Xu, M. Z.; Long, P. P.; Su, H. J.; Gan, Z. H.; Yu, Q. S. CD44-targeted facile enzymatic activatable chitosan nanoparticles for efficient antitumor therapy and reversal of multidrug resistance. Biomacromolecules 2018, 19, 883–895.
Singer, J. W.; Baker, B.; de Vries, P.; Kumar, A.; Shaffer, S.; Vawter, E.; Bolton, M.; Garzone, P. Poly-(L)-glutamic acid-paclitaxel (CT-2103)[XYOTAX™], a biodegradable polymeric drug conjugate. In Polymer Drugs in the Clinical Stage; Maeda, H.; Kabanov, A.; Kataoka, K.; Okano, T., Eds.; Springer: Boston, 2004; pp 81–99.
Langer, C. J. CT-2103: A novel macromolecular taxane with potential advantages compared with conventional taxanes. Clin. Lung Cancer 2004, 6, S85–S88.
De Jesús, O. L. P.; Ihre, H. R.; Gagne, L.; Fréchet, J. M. J.; Szoka, F. C. Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjugate Chem. 2002, 13, 453–461.
Satsangi, A.; Roy, S. S.; Satsangi, R. K.; Vadlamudi, R. K.; Ong, J. L. Design of a paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cells. Mol. Pharmaceutics 2014, 11, 1906–1918.
Satsangi, A.; Roy, S. S.; Satsangi, R. K.; Tolcher, A. W.; Vadlamudi, R. K.; Goins, B.; Ong, J. L. Synthesis of a novel, sequentially active-targeted drug delivery nanoplatform for breast cancer therapy. Biomaterials 2015, 59, 88–101.
Calderón, M.; Graeser, R.; Kratz, F.; Haag, R. Development of enzymatically cleavable prodrugs derived from dendritic polyglycerol. Bioorg. Med. Chem. Lett. 2009, 19, 3725–3728.
Malik, N.; Evagorou, E. G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anticancer Drugs 1999, 10, 767–776.
Etrych, T.; Strohalm, J.; Chytil, P.; Černoch, P.; Starovoytova, L.; Pechar, M.; Ulbrich, K. Biodegradable star HPMA polymer conjugates of doxorubicin for passive tumor targeting. Eur. J. Pharm. Sci. 2011, 42, 527–539.
Lee, S. J.; Jeong, Y. I.; Park, H. K.; Kang, D. H.; Oh, J. S.; Lee, S. G.; Lee, H. C. Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int. J. Nanomedicine 2015, 10, 5489–5503.
Zhang, C. Y.; Pan, D. Y.; Luo, K.; Li, N.; Guo, C. H.; Zheng, X. L.; Gu, Z. W. Dendrimer-doxorubicin conjugate as enzyme-sensitive and polymeric nanoscale drug delivery vehicle for ovarian cancer therapy. Polym. Chem. 2014, 5, 5227–5235.
Zhang, C. Y.; Pan, D. Y.; Li, J.; Hu, J. N.; Bains, A.; Guys, N.; Zhu, H. Y.; Li, X. H.; Luo, K.; Gong, Q. Y. et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162.
Stein, E. M.; Stein, A.; Walter, R. B.; Fathi, A. T.; Lancet, J. E.; Kovacsovics, T. J.; Advani, A. S.; DeAngelo, D. J.; O’Meara, M. M.; Zhao, B. T. et al. Interim analysis of a phase 1 trial of SGN-CD33A in patients with CD33-positive acute myeloid leukemia (AML). Blood 2014, 124, 623.
Li, N.; Cai, H.; Jiang, L.; Hu, J. N.; Bains, A.; Hu, J.; Gong, Q. Y.; Luo, K.; Gu, Z. W. Enzyme-sensitive and amphiphilic PEGylated dendrimer-paclitaxel prodrug-based nanoparticles for enhanced stability and anticancer efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877.
Chau, C. H.; Steeg, P. S.; Figg, W. D. Antibody-drug conjugates for cancer. Lancet 2019, 394, 793–804.
Lu, J.; Jiang, F.; Lu, A. P.; Zhang, G. Linkers having a crucial role in antibody-drug conjugates. Int. J. Mol. Sci. 2016, 17, 561.
McCombs, J. R.; Owen, S. C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351.
Bargh, J. D.; Isidro-Llobet, A.; Parker, J. S.; Spring, D. R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374.
Tsuchikama, K.; An, Z. Q. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46.
Mondal, D.; Ford, J.; Pinney, K. G. Improved methodology for the synthesis of a cathepsin B cleavable dipeptide linker, widely used in antibody-drug conjugate research. Tetrahedron Lett. 2018, 59, 3594–3599.
Yao, H. Z.; Jiang, F.; Lu, A. P.; Zhang, G. Methods to design and synthesize antibody-drug conjugates (ADCs). Int. J. Mol. Sci. 2016, 17, 194.
Gikanga, B.; Adeniji, N. S.; Patapoff, T. W.; Chih, H. W.; Yi, L. Cathepsin B cleavage of vcMMAE-based antibody-drug conjugate is not drug location or monoclonal antibody carrier specific. Bioconjugate Chem. 2016, 27, 1040–1049.
Boylan, N. J.; Zhou, W.; Proos, R. J.; Tolbert, T. J.; Wolfe, J. L.; Laurence, J. S. Conjugation site heterogeneity causes variable electrostatic properties in Fc conjugates. Bioconjugate Chem. 2013, 24, 1008–1016.
Poudel, Y. B.; Chowdari, N. S.; Cheng, H.; Iwuagwu, C. I.; King, H. D.; Kotapati, S.; Passmore, D.; Rampulla, R.; Mathur, A.; Vite, G. et al. Chemical modification of linkers provides stable linker-payloads for the generation of antibody-drug conjugates. ACS Med. Chem. Lett. 2020, 11, 2190–2194.
Wei, B. Q.; Gunzner-Toste, J.; Yao, H.; Wang, T.; Wang, J.; Xu, Z. J.; Chen, J. H.; Wai, J.; Nonomiya, J.; Tsai, S. P. et al. Discovery of peptidomimetic antibody-drug conjugate linkers with enhanced protease specificity. J. Med. Chem. 2018, 61, 989–1000.
Kern, J. C.; Dooney, D.; Zhang, R. N.; Liang, L. D.; Brandish, P. E.; Cheng, M. G.; Feng, G.; Beck, A.; Bresson, D.; Firdos, J. et al. Novel phosphate modified cathepsin B linkers: Improving aqueous solubility and enhancing payload scope of ADCs. Bioconjugate Chem. 2016, 27, 2081–2088.
Chen, H.; Lin, Z. T.; Arnst, K. E.; Miller, D. D.; Li, W. Tubulin inhibitor-based antibody-drug conjugates for cancer therapy. Molecules 2017, 22, 1281.
Birrer, M. J.; Moore, K. N.; Betella, I.; Bates, R. C. Antibody-drug conjugate-based therapeutics: State of the science. JNCI:J. Natl. Cancer Inst. 2019, 111, 538–549.
Chowdari, N. S.; Pan, C.; Rao, C.; Langley, D. R.; Sivaprakasam, P.; Sufi, B.; Derwin, D.; Wang, Y. C.; Kwok, E.; Passmore, D. et al. Uncialamycin as a novel payload for antibody drug conjugate (ADC) based targeted cancer therapy. Bioorg. Med. Chem. Lett. 2019, 29, 466–470.
Poudel, Y. B.; Rao, C.; Kotapati, S.; Deshpande, M.; Thevanayagam, L.; Pan, C.; Cardarelli, J.; Chowdari, N.; Kaspady, M.; Samikannu, R. et al. Design, synthesis and biological evaluation of phenol-linked uncialamycin antibody-drug conjugates. Bioorg. Med. Chem. Lett. 2020, 30, 126782.
Lim, R. K. V.; Yu, S.; Cheng, B.; Li, S. J.; Kim, N. J.; Cao, Y.; Chi, V.; Kim, J. Y.; Chatterjee, A. K.; Schultz, P. G. et al. Targeted delivery of LXR agonist using a site-specific antibody-drug conjugate. Bioconjugate Chem. 2015, 26, 2216–2222.
Kemp, G. C.; Tiberghien, A. C.; Patel, N. V.; D’Hooge, F.; Nilapwar, S. M.; Adams, L. R.; Corbett, S.; Williams, D. G.; Hartley, J. A.; Howard, P. W. Synthesis and in vitro evaluation of SG3227, a pyrrolobenzodiazepine dimer antibody-drug conjugate payload based on sibiromycin. Bioorg. Med. Chem. Lett. 2017, 27, 1154–1158.
Tiberghien, A. C.; Levy, J. N.; Masterson, L. A.; Patel, N. V.; Adams, L. R.; Corbett, S.; Williams, D. G.; Hartley, J. A.; Howard, P. W. Design and synthesis of tesirine, a clinical antibody-drug conjugate pyrrolobenzodiazepine dimer payload. ACS Med. Chem. Lett. 2016, 7, 983–987.
Smith, S. W.; Jammalamadaka, V.; Borkin, D.; Zhu, J. Y.; Degrado, S. J.; Lu, J.; Huang, J. Q.; Jiang, Y. P.; Jain, N.; Junutula, J. R. Design and synthesis of isoquinolidinobenzodiazepine dimers, a novel class of antibody-drug conjugate payload. ACS Med. Chem. Lett. 2018, 9, 56–60.
Oflazoglu, E.; Kissler, K. M.; Sievers, E. L.; Grewal, I. S.; Gerber, H. P. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br. J. Haematol. 2008, 142, 69–73.
McCombs, J. R.; Chang, H. P.; Shah, D. K.; Owen, S. C. Antibody-drug conjugate and free geldanamycin combination therapy enhances anti-cancer efficacy. Int. J. Pharm. 2021, 610, 121272.
Xiao, D.; Zhao, L.; Xie, F.; Fan, S. Y.; Liu, L. Q.; Li, W.; Cao, R. Y.; Li, S.; Zhong, W.; Zhou, X. B. A bifunctional molecule-based strategy for the development of theranostic antibody-drug conjugate. Theranostics 2021, 11, 2550–2563.
Eoin, F.; Shankar, S.; Robert, C. M.; Masahiro, N.; Christian, R. H. R.; Takao, S.; Feiedrich, S.; Gerrit, V. M.; Michael, J. O. W.; Edward, A. D. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14.
Signorell, R. D.; Luciani, P.; Brambilla, D.; Leroux, J. C. Pharmacokinetics of lipid-drug conjugates loaded into liposomes. Eur. J. Pharm. Biopharm. 2018, 128, 188–199.
Mura, S.; Bui, D. T.; Couvreur, P.; Nicolas, J. Lipid prodrug nanocarriers in cancer therapy. J. Control. Release 2015, 208, 25–41.
Maksimenko, A.; Mougin, J.; Mura, S.; Sliwinski, E.; Lepeltier, E.; Bourgaux, C.; Lepêtre, S.; Zouhiri, F.; Desmaële, D.; Couvreur, P. Polyisoprenoyl gemcitabine conjugates self assemble as nanoparticles, useful for cancer therapy. Cancer Lett. 2013, 334, 346–353.
Immordino, M. L.; Brusa, P.; Rocco, F.; Arpicco, S.; Ceruti, M.; Cattel, L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Control. Release 2004, 100, 331–346.
Bulanadi, J. C.; Xue, A. Q.; Gong, X. J.; Bean, P. A.; Julovi, S. M.; de Campo, L.; Smith, R. C.; Moghaddam, M. J. Biomimetic gemcitabine-lipid prodrug nanoparticles for pancreatic cancer. ChemPlusChem 2020, 85, 1283–1291.
Gaudin, A.; Song, E.; King, A. R.; Saucier-Sawyer, J. K.; Bindra, R.; Desmaële, D.; Couvreur, P.; Saltzman, W. M. PEGylated squalenoyl-gemcitabine nanoparticles for the treatment of glioblastoma. Biomaterials 2016, 105, 136–144.
Coppens, E.; Desmaële, D.; Mougin, J.; Tusseau-Nenez, S.; Couvreur, P.; Mura, S. Gemcitabine lipid prodrugs: The key role of the lipid moiety on the self-assembly into nanoparticles. Bioconjugate Chem. 2021, 32, 782–793.
Wu, L. M.; Zhang, F.; Chen, X. N.; Wan, J. Q.; Wang, Y. C.; Li, T. Y.; Wang, H. X. Self-assembled gemcitabine prodrug nanoparticles show enhanced efficacy against patient-derived pancreatic ductal adenocarcinoma. ACS Appl. Mater. Interfaces 2020, 12, 3327–3340.
Li, Y.; Lin, J. Y.; Wu, H. J.; Chang, Y.; Yuan, C. H.; Liu, C.; Wang, S.; Hou, Z. Q.; Dai, L. Z. Orthogonally functionalized nanoscale micelles for active targeted codelivery of methotrexate and mitomycin C with synergistic anticancer effect. Mol. Pharmaceutics 2015, 12, 769–782.
Maksimenko, A.; Alami, M.; Zouhiri, F.; Brion, J. D.; Pruvost, A.; Mougin, J.; Hamze, A.; Boissenot, T.; Provot, O.; Desmaële, D. et al. Therapeutic modalities of squalenoyl nanocomposites in colon cancer: An ongoing search for improved efficacy. ACS Nano 2014, 8, 2018–2032.
Ding, Y.; Sun, Z. Q.; Tong, Z. R.; Zhang, S. T.; Min, J.; Xu, Q. H.; Zhou, L. Z.; Mao, Z. W.; Xia, H. B.; Wang, W. L. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics 2020, 10, 5195–5208.
Ye, W. Y.; Han, H. J.; Li, H.; Jin, Q.; Wu, Y. Z.; Chakrabortty, S.; Weil, T.; Ji, J. Polymer coated nanodiamonds as gemcitabine prodrug with enzymatic sensitivity for pancreatic cancer treatment. Prog. Nat. Sci. Mater. Int. 2020, 30, 711–717.
Yang, Y. M.; Aw, J.; Chen, K.; Liu, F.; Padmanabhan, P.; Hou, Y. L.; Cheng, Z.; Xing, B. G. Enzyme-responsive multifunctional magnetic nanoparticles for tumor intracellular drug delivery and imaging. Chem.—Asian J. 2011, 6, 1381–1389.
Han, H. J.; Valdepérez, D.; Jin, Q.; Yang, B.; Li, Z. H.; Wu, Y. L.; Pelaz, B.; Parak, W. J.; Ji, J. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano 2017, 11, 1281–1291.
Zhang, H. J.; Zhao, X.; Chen, L. J.; Yang, C. X.; Yan, X. P. pH-driven targeting nanoprobe with dual-responsive drug release for persistent luminescence imaging and chemotherapy of tumor. Anal. Chem. 2019, 92, 1179–1188.
Cheng, Y. J.; Luo, G. F.; Zhu, J. Y.; Xu, X. D.; Zeng, X.; Cheng, D. B.; Li, Y. M.; Wu, Y.; Zhang, X. Z.; Zhuo, R. X. et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087.
de la Torre, C.; Mondragón, L.; Coll, C.; Sancenón, F.; Marcos, M. D.; Martínez-Máñez, R.; Amorós, P.; Pérez-Payá, E.; Orzáez, M. Cathepsin-B induced controlled release from peptide-capped mesoporous silica nanoparticles. Chem.—Eur.J. 2014, 20, 15309–15314.
Zheng, F. F.; Zhang, P. H.; Xi, Y.; Huang, K. K.; Min, Q. H.; Zhu, J. J. Peptide-mediated core/satellite/shell multifunctional nanovehicles for precise imaging of cathepsin B activity and dualenzyme controlled drug release. NPG Asia Mater. 2017, 9, e366.
Vasey, P. A.; Kaye, S. B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A. H.; Murray, L. S.; Hilditch, T. E.; Murray, T. et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents—Drug-polymer conjugates. Clin. Cancer Res. 1999, 5, 83–94.
Seymour, L. W.; Ferry, D. R.; Anderson, D.; Hesslewood, S.; Julyan, P. J.; Doran, R. P.; Young, A. M.; Burtles, S.; Kerr, D. J. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676.
Rademaker-Lakhai, J. M.; Terret, C.; Howell, S. B.; Baud, C. M.; de Boer, R. F.; Pluim, D.; Beijnen, J. H.; Schellens, J. H. M.; Droz, J. P. A phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin. Cancer Res. 2004, 10, 3386–3395.
Boddy, A. V.; Plummer, E. R.; Todd, R.; Sludden, J.; Griffin, M.; Robson, L.; Cassidy, J.; Bissett, D.; Bernareggi, A.; Verrill, M. W. et al. A phase I and pharmacokinetic study of paclitaxel poliglumex (XYOTAX), investigating both 3-weekly and 2-weekly schedules. Clin. Cancer Res. 2005, 11, 7834–7840.
Singer, J. W. Paclitaxel poliglumex (XYOTAX™, CT-2103): A macromolecular taxane. J. Control. Release 2005, 109, 120–126.
Williams, R. Discontinued drugs in 2008: Oncology drugs. Expert Opin. Investig. Drugs 2009, 13, 1581–1594.
Miller, K.; Erez, R.; Segal, E.; Shabat, D.; Satchi-Fainaro, R. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugate. Angew. Chem. 2009, 121, 2993–2998.
Younes, A.; Yasothan, U.; Kirkpatrick, P. Brentuximab vedotin. Nat. Rev. Drug Discov. 2012, 11, 19–20.
Deeks, E. D. Polatuzumab vedotin: First global approval. Drugs 2019, 79, 1467–1475.
Shultes, K. C. Polatuzumab vedotin-piiq (Polivy®). Oncol. Times 2020, 42, 9.
Petrylak, D. P.; Perez, R. P.; Zhang, J. S.; Smith, D. C.; Ruether, J. D.; Sridhar, S. S.; Sangha, R. S.; Lang, J. M.; Heath, E. I.; Merchan, J. R. et al. A phase I study of enfortumab vedotin (ASG-22CE; ASG-22ME): Updated analysis of patients with metastatic urothelial cancer. J. Chin. Oncol. 2017, 35, 106.
Chang, E.; Weinstock, C.; Zhang, L. J.; Charlab, R.; Dorff, S. E.; Gong, Y. T.; Hsu, V.; Li, F.; Ricks, T. K.; Song, P. F. et al. FDA approval summary: Enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 2021, 27, 922–927.
Coleman, R. L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F. et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619.
Teicher, B. A.; Chari, R. V. J. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397.
Yang, S.; Shim, M. K.; Kim, W. J.; Choi, J.; Nam, G. H.; Kim, J.; Kim, J.; Moon, Y.; Kim, H. Y.; Park, J. et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials 2021, 272, 120791.
Kim, J.; Shim, M. K.; Yang, S.; Moon, Y.; Song, S.; Choi, J.; Kim, J.; Kim, K. Combination of cancer-specific prodrug nanoparticle with Bcl-2 inhibitor to overcome acquired drug resistance. J. Control. Release 2021, 330, 920–932.
Shim, M. K.; Moon, Y.; Yang, S.; Kim, J.; Cho, H.; Lim, S.; Yoon, H. Y.; Seong, J. K.; Kim, K. Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials 2020, 261, 120347.
Choi, J.; Shim, M. K.; Yang, S.; Hwang, H. S.; Cho, H.; Kim, J.; Yun, W. S.; Moon, Y.; Kim, J.; Yoon, H. Y. et al. Visible-light-triggered prodrug nanoparticles combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. ACS Nano 2021, 15, 12086–12098.
Doronina, S. O.; Toki, B. E.; Torgov, M. Y.; Mendelsohn, B. A.; Cerveny, C. G.; Chace, D. F.; DeBlanc, R. L.; Gearing, R. P.; Bovee, T. D.; Siegall, C. B. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003, 21, 778–784.
Banerjee, S.; Oza, A. M.; Birrer, M. J.; Hamilton, E. P.; Hasan, J.; Leary, A.; Moore, K. N.; Mackowiak-Matejczyk, B.; Pikiel, J.; Ray-Coquard, I. et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann. Oncol. 2018, 29, 917–923.
Paz-Ares, L.; Ross, H.; O’Brien, M.; Riviere, A.; Gatzemeier, U.; Von Pawel, J.; Kaukel, E.; Freitag, L.; Digel, W.; Bischoff, H. et al. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br. J. Cancer 2008, 98, 1608–1613.
Tong, R.; Cheng, J. J. Anticancer polymeric nanomedicines. Polym. Rev. 2007, 47, 345–381.
O’Brien, M. E. R.; Socinski, M. A.; Popovich, A. Y.; Bondarenko, I. N.; Tomova, A.; Bilynskyĭ, B. T.; Hotko, Y. S.; Ganul, V. L.; Kostinsky, I. Y.; Eisenfeld, A. J. et al. Randomized phase III trial comparing single-agent paclitaxel poliglumex (CT-2103, PPX) with single-agent gemcitabine or vinorelbine for the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 728–734.
Markham, A. Tisotumab vedotin: First approval. Drugs 2021, 31, 2141–2147.
Keam, S. J. Trastuzumab deruxtecan: First approval. Drugs 2020, 80, 501–508.
Manich, C. S.; O’Shaughnessy, J.; Aftimos, P. G.; van den Tweel, E.; Oesterholt, M.; Escrivá-de-Romaní, S. I.; Tueux, N. Q.; Tan, T. J.; Lim, J. S.; Ladoire, S. et al. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-] trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 2021, 32, S1288.
Bendell, J.; Saleh, M.; Rose, A. A. N.; Siegel, P. M.; Hart, L.; Sirpal, S.; Jones, S.; Green, J.; Crowley, E.; Simantov, R. et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3619–3625.
Mullard, A. Cancer stem cell candidate Rova-T discontinued. Nat. Rev. Drug Discov. 2019, 13, 814.
Petrylak, D. P.; Smith, D. C.; Appleman, L. J.; Fleming, M. T.; Hussain, A.; Dreicer, R.; Sartor, A. O.; Shore, N. D.; Vogelzang, N. J.; Youssoufian, H. et al. A phase II trial of prostate-specific membrane antigen antibody drug conjugate (PSMA ADC) in taxane-refractory metastatic castration-resistant prostate cancer (mCRPC). J. Chin. Oncol. 2014, 32, 83.
Waqar, S. N.; Redman, M. W.; Arnold, S. M.; Hirsch, F. R.; Mack, P. C.; Schwartz, L. H.; Gandara, D. R.; Stinchcombe, T. E.; Leighl, N. B.; Ramalingam, S. S. et al. A phase II study of telisotuzumab vedotin in patients with c-MET-positive stage IV or recurrent squamous cell lung cancer (LUNG-MAP Sub-study S1400K, NCT03574753). Clin. Lung Cancer 2021, 22, 170–177.
Han, H. S.; Alemany, C. A.; Brown-Glaberman, U. A.; Pluard, T. J.; Sinha, R.; Sterrenberg, D.; Albain, K. S.; Basho, R. K.; Biggs, D.; Boni, V. et al. SGNLVA-002: Single-arm, open label phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer. J. Clin. Oncol. 2019, 37.
Zambrano, C. C.; Almhanna, K.; Messersmith, W. A.; Ahnert, J. R.; Ryan, D. P.; Faris, J. E.; Jung, J. A.; Fasanmade, A.; Wyant, T.; Kalebic, T. MLN0264, an investigational antiguanylyl cyclase C (GCC) antibody-drug conjugate (ADC), in patients (pts) with advanced gastrointestinal (GI) malignancies: Phase I study. J. Clin. Oncol. 2014, 32, 3546.
Advani, R. H.; Lebovic, D.; Chen, A.; Brunvand, M.; Goy, A.; Chang, J. E.; Hochberg, E.; Yalamanchili, S.; Kahn, R.; Lu, D. et al. Phase I study of the anti-CD22 antibody-drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2017, 23, 1167–1176.
Sawas, A.; Savage, K. J.; Perez, R.; Advani, R. H.; Butturini, A.; Lackey, J.; Trave, F.; Anand, B.; Huang, Y.; Reyno, L. et al. A phase 1 study of the anti-CD37 antibody-drug conjugate AGS67E in advanced lymphoid malignancies. Interim results. Blood 2015, 126, 3976.
Petrylak, D.; Heath, E.; Sonpavde, G.; George, S.; Morgans, A. K.; Eigl, B. J.; Picus, J.; Cheng, S.; Hotte, S. J.; Gartner, E. et al. Interim analysis of a phase I dose escalation trial of the antibody drug conjugate (ADC) AGS15E (ASG-15ME) in patients (Pts) with metastatic urothelial cancer (mUC). Ann. Oncol. 2016, 27, vi269.
Owonikoko, T. K.; Hussain, A.; Stadler, W. M.; Smith, D. C.; Kluger, H.; Molina, A. M.; Gulati, P.; Shah, A.; Ahlers, C. M.; Cardarelli, P. M. et al. First-in-human multicenter phase I study of BMS-936561 (MDX-1203), an antibody-drug conjugate targeting CD70. Cancer Chemother. Pharmacol. 2016, 77, 155–162.
Danila, D. C.; Szmulewitz, R. Z.; Baron, A. D.; Higano, C. S.; Scher, H. I.; Morris, M. J.; Gilbert, H.; Brunstein, F.; Lemahieu, V.; Kabbarah, O. et al. A phase I study of DSTP3086S, an antibody-drug conjugate (ADC) targeting STEAP-1, in patients (pts) with metastatic castration-resistant prostate cancer (CRPC). J. Clin. Oncol. 2014, 32, 5024.
Liu, J. F.; Moore, K. N.; Birrer, M. J.; Berlin, S.; Matulonis, U. A.; Infante, J. R.; Wolpin, B.; Poon, K. A.; Firestein, R.; Xu, J. et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann. Oncol. 2016, 27, 2124–2130.
Phillips, T.; Barr, P. M.; Park, S. I.; Kolibaba, K.; Caimi, P. F.; Chhabra, S.; Kingsley, E. C.; Boyd, T.; Chen, R.; Carret, A. S. et al. A phase 1 trial of SGN-CD70A in patients with CD70-positive diffuse large B cell lymphoma and mantle cell lymphoma. Invest. New Drugs 2019, 37, 297–306.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Nos. NRF-2019R1A2C3006283 and NRF-2021R1C1C2005460), the KU-KIST Graduate School of Converging Science and Technology (Korea University & KIST), and the Intramural Research Program of KIST.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeon, S.I., Yang, S., Shim, M.K. et al. Cathepsin B-responsive prodrugs for cancer-targeted therapy: Recent advances and progress for clinical translation. Nano Res. 15, 7247–7266 (2022). https://doi.org/10.1007/s12274-022-4354-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4354-y