Abstract
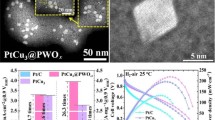
Low-platinum (Pt) alloy catalysts hold promising application in oxygen reduction reaction (ORR) electrocatalysis of proton-exchange-membrane fuel cells (PEMFCs). Although significant progress has been made to boost the kinetic ORR mass activity at low current densities in liquid half-cells, little attention was paid to the performance of Pt-based catalysts in realistic PEMFCs particularly at high current densities for high power density, which remains poorly understood. In this paper, we show that, regardless of the kinetic mass activity at the low current density region, the high current density performance of the low-Pt alloy catalysts is dominantly controlled by the total Pt surface area, particularly in low-Pt-loading H2-air PEMFCs. To this end, we propose two different strategies to boost the specific Pt surface area, the post-15-nm dealloyed nanoporous architecture and the sub-5-nm solid core-shell nanoparticles (NPs) through fluidic-bed synthesis, both of which bring in comparably high mass activity and high Pt surface area for large-current-density performance. At medium current density, the dealloyed porous NPs provide substantially higher H2-air PEMFC performance compared to solid core-shell catalysts, despite their similar mass activity in liquid half-cells. Scanning transmission electron microscopy images combined with electron energy loss spectroscopic imaging evidence a previously unreported “semi-immersed nanoporous-Pt/ionomer” structure in contrast to a “fully-immersed core-shell-Pt/ionomer” structure, thus favoring O2 transport and improving the fuel cell performance. Our results provide new insights into the role of Pt nanostructures in concurrently optimizing the mass activity, Pt surface area and Pt/Nafion interface for high power density fuel cells.

Similar content being viewed by others
References
Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147.
Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C. F.; Liu, Z. C.; Kaya, S.; Nordlund, D.; Ogasawara, H. et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460.
Gan, L.; Heggen, M.; O’Malley, R.; Theobald, B.; Strasser, P. Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 2013, 13, 1131–1138.
Wang, R. Y.; Xu, C. X.; Bi, X. X.; Ding, Y. Nanoporous surface alloys as highly active and durable oxygen reduction reaction electrocatalysts. Energy Environ. Sci. 2012, 5, 5281–5286.
Li, J.; Yin, H. M.; Li, X. B.; Okunishi, E.; Shen, Y. L.; He, J.; Tang, Z. K.; Wang, W. X.; Yücelen, E.; Li, C. et al. Surface evolution of a Pt−Pd−Au electrocatalyst for stable oxygen reduction. Nat. Energy 2017, 2, 17111.
Tian, X. L.; Zhao, X.; Su, Y. Q.; Wang, L. J; Wang, H. M.; Dang, D.; Chi, B.; Liu, H. F.; Hensen, E. J. M.; Lou, X. W. et al. Engineering bunched Pt−Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856.
Cui, C. H.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771.
Yang, F.; Ye, J. Y.; Yuan, Q.; Yang, X. T.; Xie, Z. X.; Zhao, F. L.; Zhou, Z. Y.; Gu, L.; Wang, X. Ultrasmall Pd−Cu−Pt trimetallic twin icosahedrons boost the electrocatalytic performance of glycerol oxidation at the operating temperature of fuel cells. Adv. Funct. Mater. 2020, 30, 1908235.
Chen, C.; Kang, Y. J.; Huo, Z. Y.; Zhu, Z. W.; Huang, W. Y.; Xin, H. L.; Snyder, J. D.; Li, D. G.; Herron, J. A.; Mavrikakis, M. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Chen, S. P.; Li, M. F.; Gao, M. Y.; Jin, J. B.; Van Spronsen, M. A.; Salmeron, M. B.; Yang, P. D. High-performance Pt−Co nanoframes for fuel-cell electrocatalysis. Nano Lett. 2020, 20, 1974–1979.
Lei, W. J.; Li, M. G.; He, L.; Meng, X.; Mu, Z. J.; Yu, Y. S.; Ross, F. M.; Yang, W. W. A general strategy for bimetallic Pt-based nanobranched structures as highly active and stable oxygen reduction and methanol oxidation bifunctional catalysts. Nano Res. 2020, 13, 638–645.
Wang, Z. X.; Yao, X. Z.; Kang, Y. Q.; Miao, L. Q.; Xia, D. S.; Gan, L. Structurally ordered low-Pt intermetallic electrocatalysts toward durably high oxygen reduction reaction activity. Adv. Funct. Mater. 2019, 29, 1902987.
Zhao, T.; Luo, E. G.; Li, Y.; Wang, X.; Liu, C. P.; Xing, W.; Ge, J. J. Highly dispersed L10−PtZn intermetallic catalyst for efficient oxygen reduction. Sci. China Mater. 2021, 64, 1671–1678.
Zhao, F. L.; Zheng, L. R.; Yuan, Q.; Yang, X. T.; Zhang, Q. H.; Xu, H.; Guo, Y. L.; Yang, S.; Zhou, Z. Y.; Gu, L. et al. Ultrathin PdAuBiTe nanosheets as high-performance oxygen reduction catalysts for a direct methanol fuel cell device. Adv. Mater. 2021, 33, 2103383.
Stephens, I. E. L.; Rossmeisl, J.; Chorkendorff, I. Toward sustainable fuel cells. Science 2016, 354, 1378–1379.
Fan, J. T.; Chen, M.; Zhao, Z. L.; Zhang, Z.; Ye, S. Y.; Xu, S. Y.; Wang, H. J.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486.
Han, B. H.; Carlton, C. E.; Kongkanand, A.; Kukreja, R. S.; Theobald, B. R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F. T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266.
Stamenkovic, V. R.; Markovic, N. M. Nanosegregated cathode catalysts with ultra-low platinum loading. In 2015 DOE Hydrogen and Fuel Cells Program Review; U. S. Department of Energy: Washington, 2015. https://www.hydrogen.energy.gov/pdfs/review15/fc008_stamenkovic_2015_o.pdf (accessed Jun 8, 2015)
Kongkanand, A.; Mathias, M. F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137.
Kongkanand, A.; Subramanian, N. P.; Yu, Y. C.; Liu, Z. Y.; Igarashi, H.; Muller, D. A. Achieving high-power PEM fuel cell performance with an ultralow-Pt-content core-shell catalyst. ACS Catal. 2016, 6, 1578–1583.
Li, M. F.; Zhao, Z. P.; Cheng, T.; Fortunelli, A.; Chen, C. Y.; Yu, R.; Zhang, Q. H.; Gu, L.; Merinov, B. V.; Lin, Z. Y. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419.
Liang, J. S.; Li, N.; Zhao, Z. L.; Ma, L.; Wang, X. M.; Li, S. Z.; Liu, X.; Wang, T. Y.; Du, Y. P.; Lu, G. et al. Tungsten-doped L10−PtCo ultrasmall nanoparticles as a high-performance fuel cell cathode. Angew. Chem., Int. Ed. 2019, 58, 15471–15477.
Liang, J. S.; Zhao, Z. L.; Li, N.; Wang, X. M.; Li, S. Z.; Liu, X.; Wang, T. Y.; Lu, G.; Wang, D. L.; Hwang, B. J. et al. Biaxial strains mediated oxygen reduction electrocatalysis on fenton reaction resistant L10−PtZn fuel cell cathode. Adv. Energy Mater. 2020, 10, 2000179.
Yang, Z. J.; Yang, H. Z.; Shang, L.; Zhang, T. R. Ordered PtFeIr intermetallic nanowires prepared through a silica-protection strategy for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2022, 61, e202113278.
Cui, Y. F.; Wu, Y. L.; Wang, Z. X.; Yao, X. Z.; Wei, Y. P.; Kang, Y. Q.; Du, H. D.; Li, J.; Gan, L. Mitigating metal dissolution and redeposition of Pt−Co catalysts in pem fuel cells: Impacts of structural ordering and particle size. J. Electrochem. Soc. 2020, 167, 064520.
Ramaswamy, N.; Gu, W. B.; Ziegelbauer, J. M.; Kumaraguru, S. Carbon support microstructure impact on high current density transport resistances in pemfc cathode. J. Electrochem. Soc. 2020, 167, 064515.
Yarlagadda, V.; Carpenter, M. K.; Moylan, T. E.; Kukreja, R. S.; Koestner, R.; Gu, W. B.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621.
Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H. N.; Hübner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85.
Yao, X. Z.; Wei, Y. P.; Wang, Z. X.; Gan, L. Revealing the role of surface composition on the particle mobility and coalescence of carbon-supported Pt alloy fuel cell catalysts by in situ heating (S)TEM. ACS Catal. 2020, 10, 7381–7388.
Hornberger, E.; Schmies, H.; Paul, B.; Kühl, S.; Strasser, P. Design and validation of a fluidized bed catalyst reduction reactor for the synthesis of well-dispersed nanoparticle ensembles. J. Electrochem. Soc. 2020, 167, 114509.
Oezaslan, M.; Heggen, M.; Strasser, P. Size-dependent morphology of dealloyed bimetallic catalysts: Linking the nano to the macro scale. J. Am. Chem. Soc. 2012, 134, 514–524.
Gan, L.; Cui, C. H.; Rudi, S.; Strasser, P. Core-shell and nanoporous particle architectures and their effect on the activity and stability of Pt ORR electrocatalysts. Top. Catal. 2014, 57, 236–244.
Wang, J. X.; Uribe, F. A.; Springer, T. E.; Zhang, J. L.; Adzic, R. R. Intrinsic kinetic equation for oxygenreduction reaction in acidic media: The double tafel slope and fuelcell applications. Faraday Discuss. 2009, 140, 347–362.
Holewinski, A.; Linic, S. Elementary mechanisms in electrocatalysis: Revisiting the ORR tafel slope. J. Electrochem. Soc. 2012, 159, H864–H870.
Shinagawa, T.; Garcia-Esparza, A. T.; Takanabe, K. Insight on tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801.
Gasteiger, H. A.; Kocha, S. S.; Sompalli, B.; Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B: Environ. 2005, 56, 9–35.
Wu, B. B.; Li, B.; Liu, W. M.; Liu, J. G.; Zhao, M.; Yao, Y. F.; Gu, J.; Zou, Z. G. The performance improvement of membrane and electrode assembly in open-cathode proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2013, 38, 10978–10984.
Lim, B. H.; Majlan, E. H.; Tajuddin, A.; Husaini, T.; Wan Daud, W. R.; Mohd Radzuan, N. A.; Haque, M. A. Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly: A review. Chin. J. Chem. Eng. 2021, 33, 1–16.
Asghari, S.; Mokmeli, A.; Samavati, M. Study of PEM fuel cell performance by electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2010, 35, 9283–9290.
Pang, J.; Li, S.; Wang, R. Y.; Zhu, K.; Liu, S. C.; Guo, Z.; Pan, M. Durability failure analysis of proton exchange membrane fuel cell and its effect on oxygen transport in gas diffusion layer. J. Electrochem. Soc. 2019, 166, F1016–F1021.
Mack, F.; Laukenmann, R.; Galbiati, S.; Kerres, J. A.; Zeis, R. Electrochemical impedance spectroscopy as a diagnostic tool for high-temperature PEM fuel cells. ECS Trans. 2015, 69, 1075–1087.
Greszler, T. A.; Caulk, D.; Sinha, P. The impact of platinum loading on oxygen transport resistance. J. Electrochem. Soc. 2012, 159, F831–F840.
Morawietz, T.; Handl, M.; Oldani, C.; Friedrich, K. A.; Hiesgen, R. Quantitative in situ analysis of ionomer structure in fuel cell catalytic layers. ACS Appl. Mater. Interfaces 2016, 8, 27044–27054.
Guan, J. Y.; Yang, S. X.; Liu, T. T.; Yu, Y. H.; Niu, J.; Zhang, Z. P.; Wang, F. Intermetallic FePt@PtBi core-shell nanoparticles for oxygen reduction electrocatalysis. Angew. Chem., Int. Ed. 2021, 60, 21899–21904.
Cheng, H.; Gui, R. J.; Yu, H.; Wang, C.; Liu, S.; Liu, H. F.; Zhou, T. P.; Zhang, N.; Zheng, X. S.; Chu, W. S. et al. Subsize Pt-based intermetallic compound enables long-term cyclic mass activity for fuel-cell oxygen reduction. Proc. Natl. Acad. Sci. USA 2021, 118, e2104026118.
Li, J. R.; Xi, Z.; Pan, Y. T.; Spendelow, J. S.; Duchesne, P. N.; Su, D.; Li, Q.; Yu, C.; Yin, Z. Y.; Shen, B. et al. Fe stabilization by intermetallic L10−FePt and Pt catalysis enhancement in L10−FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 2018, 140, 2926–2932.
Chong, L. N.; Wen, J. G.; Kubal, J.; Sen, F. G.; Zou, J. X.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W. J.; Liu, D. J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281.
Li, J. R.; Sharma, S.; Liu, X. M.; Pan, Y. T.; Spendelow, J. S.; Chi, M. F.; Jia, Y. K.; Zhang, P.; Cullen, D. A.; Xi, Z. et al. Hard-magnet L10−CoPt nanoparticles advance fuel cell catalysis. Joule 2019, 3, 124–135.
Yang, C. L.; Wang, L. N.; Yin, P.; Liu, J. Y.; Chen, M. X.; Yan, Q. Q.; Wang, Z. S.; Xu, S. L.; Chu, S. Q.; Cui, C. H. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464.
Dionigi, F.; Weber, C. C.; Primbs, M.; Gocyla, M.; Bonastre, A. M.; Spöri, C.; Schmies, H.; Hornberger, E.; Kühl, S.; Drnec, J. et al. Controlling near-surface Ni composition in octahedral PtNi(Mo) nanoparticles by Mo doping for a highly active oxygen reduction reaction catalyst. Nano Lett. 2019, 19, 6876–6885.
Debe, M. K. Tutorial on the fundamental characteristics and practical properties of nanostructured thin film (NSTF) catalysts. J. Electrochem. Soc. 2013, 160, F522–F534.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 52173222, 51622103 and 22109088), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01N111), Key Area Research and Development Program of Guangdong Province (No. 2020B0909040003), and Shenzhen Science and Technology Innovation Committee (Nos. WDZ20200819115243002 and JCYJ20190809172617313).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2022_4238_MOESM1_ESM.pdf
Engineering nanoporous and solid core-shell architectures of low-platinum alloy catalysts for high power density PEM fuel cells
Rights and permissions
About this article
Cite this article
Kang, Y., Wang, J., Wei, Y. et al. Engineering nanoporous and solid core-shell architectures of low-platinum alloy catalysts for high power density PEM fuel cells. Nano Res. 15, 6148–6155 (2022). https://doi.org/10.1007/s12274-022-4238-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4238-1