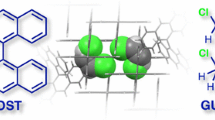
Abstract
Polycyclic aromatic hydrocarbons (PAHs) are promising nanocarbon materials with diverse optoelectronic properties, yet they also pose concerning environmental and health risks. Despite the ubiquity of PAHs in the environment (crude oil, emissions, and biomass), most supermolecules rely on heteroatoms for stability. We discovered and characterized a family of all-hydrocarbon, all-π-conjugated [n]cycloparaphenylene-PAH host-guest complexes. We built a theoretical framework to rapidly select these complexes and predict their stabilities, driven exclusively by CH−π interactions. More than a dozen complexes were confirmed experimentally and assembled directly from commercially available compounds. This motif offers a versatile way to combine the advantageous properties of organic semiconductors with the rich dynamic, stereochemical, stimulus-responsive, and stress-dissipative behavior of host-guest complexes, while creating new opportunities for bespoke PAH separation or remediation materials.

Similar content being viewed by others
References
Wu, J. S.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev.2007, 107, 718–747.
Sun, Z.; Wu, J. S. Open-shell polycyclic aromatic hydrocarbons. J. Mater. Chem.2012, 22, 4151–4160.
Kumar, R.; Aggarwal, H.; Srivastava, A. Of twists and curves: Electronics, photophysics, and upcoming applications of non-planar conjugated organic molecules. Chem.—Eur. J.2020, 26, 10653–10675.
Delgado-Saborit, J. M.; Stark, C.; Harrison, R. M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int.2011, 37, 383–392.
Collins, J. F.; Brown, J. P.; Alexeeff, G. V.; Salmon, A. G. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul. Toxicol. Pharmacol.1998, 28, 45–54.
Rieger, R.; Müllen, K. Forever young: Polycyclic aromatic hydrocarbons as model cases for structural and optical studies. J. Phys. Org. Chem.2010, 23, 315–325.
Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K. Z.; Reckmeier, C.; Rogach, A. L.; Stolarczyk, J. K.; Urban, A. S.; Feldmann, J. Carbon dots: A unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett.2015, 15, 6030–6035.
Cho, H. J.; Kim, S. W.; Kim, S.; Lee, S.; Lee, J.; Cho, Y.; Lee, Y.; Lee, T. W.; Shin, H. J.; Song, C. Suppressing π−π stacking interactions for enhanced solid-state emission of flat aromatic molecules via edge Functionalization with picket-fence-type groups. J. Mater. Chem. C2020, 8, 17289–17296.
Pisula, W.; Feng, X. L.; Müllen, K. Charge-carrier transporting graphene-type molecules. Chem. Mater.2011, 23, 554–567.
Lauchner, A.; Schlather, A. E.; Manjavacas, A.; Cui, Y.; McClain, M. J.; Stec, G. J.; De Abajo, F. J. G.; Nordlander, P.; Halas, N. J. Molecular plasmonics. Nano Lett.2015, 15, 6208–6214.
Rapenne, G.; Joachim, C. Single rotating molecule-machines: Nanovehicles and molecular motors. In Molecular Machines and Motors. Topics in Current Chemistry; Credi, A.; Silvi, S.; Venturi, M., Eds.; Springer, 2014; pp 253–277.
Steed, J. W.; Atwood, J. L. Supramolecular Chemistry; John Wiley & Sons: Chichester, 2009.
Kawase, T.; Tanaka, K.; Fujiwara, N.; Darabi, H. R.; Oda, M. Complexation of a carbon Nanoring with fullerenes. Angew. Chem., Int. Ed.2003, 42, 1624–1628.
Kawase, T.; Tanaka, K.; Shiono, N.; Seirai, Y.; Oda, M. Onion-type complexation based on carbon Nanorings and a buckminsterfullerene. Angew. Chem., Int. Ed.2004, 43, 1722–1724.
Kawase, T.; Nishiyama, Y.; Nakamura, T.; Ebi, T.; Matsumoto, K.; Kurata, H.; Oda, M. Cyclic [5]paraphenyleneacetylene: Synthesis, properties, and formation of a ring-in-ring complex showing a considerably large association constant and entropy effect. Angew. Chem., Int. Ed.2007, 46, 1086–1088.
Lee, S.; Chénard, E.; Gray, D. L.; Moore, J. S. Synthesis of cycloparaphenyleneacetylene via alkyne metathesis: C70 complexation and copper-free triple click reaction. J. Am. Chem. Soc.2016, 138, 13814–13817.
Iwamoto, T.; Watanabe, Y.; Sadahiro, T.; Haino, T.; Yamago, S. Size-selective encapsulation of C60 by [10]cycloparaphenylene: Formation of the shortest fullerene-peapod. Angew. Chem., Int. Ed.2011, 50, 8342–8344.
Iwamoto, T.; Watanabe, Y.; Takaya, H.; Haino, T.; Yasuda, N.; Yamago, S. Size- and orientation-selective encapsulation of C70 by cycloparaphenylenes. Chem.—Eur. J.2013, 19, 14061–14068.
Iwamoto, T.; Slanina, Z.; Mizorogi, N.; Guo, J. D.; Akasaka, T.; Nagase, S.; Takaya, H.; Yasuda, N.; Kato, T.; Yamago, S. Partial charge transfer in the shortest possible metallofullerene peapod, La@C82⊂[11]cycloparaphenylene. Chem.—Eur. J.2014, 20, 14403–14409.
Xia, J. L.; Bacon, J. W.; Jasti, R. Gram-scale synthesis and crystal structures of [8]- and [10]CPP, and the solid-state structure of C60@[10]CPP. Chem. Sci.2012, 3, 3018–3021.
Isobe, H.; Hitosugi, S.; Yamasaki, T.; Iizuka, R. Molecular bearings of finite carbon nanotubes and fullerenes in ensemble rolling motion. Chem. Sci.2013, 4, 1293–1297.
Sato, S.; Yamasaki, T.; Isobe, H. Solid-state structures of peapod bearings composed of finite single-wall carbon nanotube and fullerene molecules. Proc. Natl. Acad. Sci. USA2014, 111, 8374–8379.
Matsuno, T.; Sato, S.; Iizuka, R.; Isobe, H. Molecular recognition in curved π-systems: Effects of π-lengthening of tubular molecules on thermodynamics and structures. Chem. Sci.2015, 6, 909–916.
Yuan, K.; Guo, Y. J.; Zhao, X. Nature of noncovalent interactions in the [n]cycloparaphenylene⊃C70 (n = 10, 11, and 12) host-guest complexes: A theoretical insight into the shortest C70-carbon nanotube peapod. J. Phys. Chem. C2015, 119, 5168–5179.
González-Veloso, I.; Cabaleiro-Lago, E. M.; Rodríguez-Otero, J. Fullerene size controls the selective complexation of [11]CPP with pristine and endohedral fullerenes. Phys. Chem. Chem. Phys.2018, 20, 11347–11358.
Hashimoto, S.; Iwamoto, T.; Kurachi, D.; Kayahara, E.; Yamago, S. Shortest double-walled carbon nanotubes composed of cycloparaphenylenes. ChemPlusChem2017, 82, 1015–1020.
Rio, J.; Beeck, S.; Rotas, G.; Ahles, S.; Jacquemin, D.; Tagmatarchis, N.; Ewels, C.; Wegner, H. A. Electronic communication between two [10]cycloparaphenylenes and Bis(Azafullerene) (C59N)2 induced by cooperative complexation. Angew. Chem., Int. Ed.2018, 57, 6930–6934.
Yang, Y.; Juríček, M. Fullerene wires assembled inside carbon nanohoops. ChemPlusChem2022, 87, e202100468.
Jasti, R.; Bhattacharjee, J.; Neaton, J. B.; Bertozzi, C. R. Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: Carbon nanohoop structures. J. Am. Chem. Soc.2008, 130, 17646–17647.
Golder, M. R.; Jasti, R. Syntheses of the smallest carbon nanohoops and the emergence of unique physical phenomena. Acc. Chem. Res.2015, 48, 557–566.
Xu, Y. Z.; Von Delius, M. The supramolecular chemistry of strained carbon nanohoops. Angew. Chem., Int. Ed.2020, 59, 559–573.
Lu, D. P.; Huang, Q.; Wang, S. D.; Wang, J. Y.; Huang, P. S.; Du, P. W. The supramolecular chemistry of cycloparaphenylenes and their analogs. Front. Chem.2019, 7, 668.
Bachrach, S. M. Planar rings in nano-Saturns and related complexes. Chem. Commun.2019, 55, 3650–3653.
Bachrach, S. M.; Zayat, Z. C. “Planetary orbit” systems composed of cycloparaphenylenes. J. Org. Chem.2016, 81, 4559–4565.
Sala, P. D.; Talotta, C.; Caruso, T.; De Rosa, M.; Soriente, A.; Neri, P.; Gaeta, C. Tuning cycloparaphenylene host properties by chemical modification. J. Org. Chem.2017, 82, 9885–9889.
Matsuno, T.; Fujita, M.; Fukunaga, K.; Sato, S.; Isobe, H. Concyclic CH−π arrays for single-axis rotations of a bowl in a tube. Nat. Commun.2018, 9, 3779.
Adachi, S.; Shibasaki, M.; Kumagai, N. TriQuinoline. Nat. Commun.2019, 10, 3820.
Vidal-Vidal, Á.; Cabaleiro-Lago, E. M.; López, C. S.; Faza, O. N. Rational design of efficient environmental sensors: Ring-shaped nanostructures can capture Quat herbicides. ACS Omega2018, 3, 16976–16988.
Yamamoto, Y.; Tsurumaki, E.; Wakamatsu, K.; Toyota, S. Nano-Saturn: Experimental evidence of complex formation of an anthracene cyclic ring with C60. Angew. Chem., Int. Ed.2018, 57, 8199–8202.
Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm2004, 6, 130–158.
Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M. S.; Chakrabarti, P. CH−π hydrogen bonds in biological macromolecules. Phys. Chem. Chem. Phys.2014, 16, 12648–12683.
Schmidt-Mende, L.; Fechtenkötter, A.; Müllen, K.; Moons, E.; Friend, R. H.; MacKenzie, J. D. Self-organized discotic liquid crystals for high-efficiency organic photovoltaics. Science2001, 293, 1119–1122.
Xiao, S. X.; Myers, M.; Miao, Q.; Sanaur, S.; Pang, K. L.; Steigerwald, M. L.; Nuckolls, C. Molecular wires from contorted aromatic compounds. Angew. Chem., Int. Ed.2005, 44, 7390–7394.
Feng, X. L; Marcon, V.; Pisula, W.; Hansen, M. R.; Kirkpatrick, J.; Grozema, F.; Andrienko, D.; Kremer, K.; Müllen, K. Towards high charge-carrier mobilities by rational design of the shape and periphery of discotics. Nat. Mater.2009, 8, 421–426.
Sagade, A. A.; Rao, K. V.; Mogera, U.; George, S. J.; Datta, A.; Kulkarni, G. U. High-mobility field effect transistors based on supramolecular charge transfer nanofibres. Adv. Mater.2013, 25, 559–564.
Ulatowski, F.; Dąbrowa, K.; Bałakier, T.; Jurczak, J. Recognizing the limited applicability of job plots in studying host-guest interactions in supramolecular chemistry. J. Org. Chem.2016, 81, 1746–1756.
Hibbert, D. B.; Thordarson, P. The death of the job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun.2016, 52, 12792–12805.
Minameyer, M. B.; Xu, Y. Z.; Frühwald, S.; Görling, A.; Von Delius, M.; Drewello, T. Investigation of cycloparaphenylenes (CPPs) and their noncovalent ring-in-ring and fullerene-in-ring complexes by (matrix-assisted) laser desorption/ionization and density functional theory. Chem.—Eur. J.2020, 26, 8729–8741.
Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev.2011, 40, 1305–1323.
Fujitsuka, M.; Iwamoto, T.; Kayahara, E.; Yamago, S.; Majima, T. Enhancement of the quinoidal character for smaller [n]cycloparaphenylenes probed by Raman spectroscopy. ChemPhysChem2013, 14, 1570–1572.
Chen, H.; Golder, M. R.; Wang, F.; Jasti, R.; Swan, A. K. Raman spectroscopy of carbon nanohoops. Carbon2014, 67, 203–213.
Yamamoto, K.; Sonobe, H.; Matsubara, H.; Sato, M.; Okamoto, S.; Kitaura, K. Convenient new synthesis of [7]circulene. Angew. Chem., Int. Ed.1996, 35, 69–70.
Kohn, W.; Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev.1965, 140, A1133–A1138.
Anderson, J.; Burns, P. J.; Milroy, D.; Ruprecht, P.; Hauser, T.; Siegel, H. J. Deploying RMACC summit: An HPC resource for the rocky mountain region. In Proceedings of the Practice and Experience in Advanced Research Computing 2017 on Sustainability, Success and Impact, New Orleans, USA, 2017, pp 1–7.
Sousa, S. F.; Fernandes, P. A.; Ramos, M. J. General performance of density functionals. J. Phys. Chem. A2007, 111, 10439–10452.
Grimme, S.; Hansen, A.; Brandenburg, J. G.; Bannwarth, C. Dispersion-corrected mean-field electronic structure methods. Chem. Rev.2016, 116, 5105–5154.
Iikura, H.; Tsuneda, T.; Yanai, T.; Hirao, K. A long-range correction scheme for generalized-gradient-approximation exchange functionals. J. Chem. Phys.2001, 115, 3540–3544.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem.2006, 27, 1787–1799.
Verma, P.; Truhlar, D. G. Status and challenges of density functional theory. Trends Chem.2020, 2, 302–318.
Nishio, M.; Umezawa, Y.; Hirota, M.; Takeuchi, Y. The CH/π interaction: Significance in molecular recognition. Tetrahedron1995, 51, 8665–8701.
Brandl, M.; Weiss, M. S.; Jabs, A.; Suhnel, J.; Hilgenfeld, R. C-H center dot center dot center dot pi-interactions in proteins. J. Mol. Biol.2001, 307, 357–377.
Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys.2011, 13, 13873–13900.
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev.1991, 91, 893–928.
Lu, T.; Chen, F. W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem.2012, 33, 580–592.
Koch, U.; Popelier, P. L. A. Characterization of C−H−O hydrogen bonds on the basis of the charge density. J. Phys. Chem.1995, 99, 9747–9754.
Takahashi, O.; Kohno, Y.; Saito, K. Molecular orbital calculations of the substituent effect on intermolecular CH/π interaction in C2H3X−C6H6 complexes (X = H, F, Cl, Br, and OH). Chem. Phys. Lett.2003, 378, 509–515.
Connors, K. A. Binding Constants: The Measurement of Molecular Complex Stability; John Wiley & Sons: New York, 1987.
Kanagaraj, K.; Alagesan, M.; Inoue, Y.; Yang, C. Solvation effects in supramolecular chemistry. In Comprehensive Supramolecular Chemistry II; Atwood, J. L., Ed.; Elsevier: Amsterdam, 2017; pp. 11–60.
Hunter, C. A.; Anderson, H. L. What is cooperativity?. Angew. Chem., Int. Ed.2009, 48, 7488–7499.
Iwamoto, T.; Watanabe, Y.; Sakamoto, Y.; Suzuki, T.; Yamago, S. Selective and random syntheses of [n]cycloparaphenylenes (n = 8–13) and size dependence of their electronic properties. J. Am. Chem. Soc.2011, 133, 8354–8361.
Darzi, E. R.; Jasti, R. The dynamic, size-dependent properties of [5]–[12]cycloparaphenylenes. Chem. Soc. Rev.2015, 44, 6401–6410.
Hoshino, M.; Nakanishi-Ohno, Y.; Hashizume, D. Inference-assisted intelligent crystallography based on preliminary data. Sci. Rep.2019, 9, 11886.
Lin, J. B.; Darzi, E. R.; Jasti, R.; Yavuz, I.; Houk, K. N. Solid-state order and charge mobility in [5]- to [12]cycloparaphenylenes. J. Am. Chem. Soc.2019, 141, 952–960.
Salzner, U.; Aydin, A. Improved prediction of properties of π-conjugated oligomers with range-separated hybrid density functionals. J. Chem. Theory Comput.2011, 7, 2568–2583.
Matunová, P.; Jirásek, V.; Rezek, B. DFT calculations reveal pronounced HOMO-LUMO spatial separation in polypyrrole-nanodiamond systems. Phys. Chem. Chem. Phys.2019, 21, 11033–11042.
Van De Craats, A. M.; Warman, J. M. The core-size effect on the mobility of charge in discotic liquid crystalline materials. Adv. Mater.2001, 13, 130–133.
Malloci, G.; Cappellini, G.; Mulas, G.; Mattoni, A. Electronic and optical properties of families of polycyclic aromatic hydrocarbons: A systematic (time-dependent) density functional theory study. Chem. Phys.2011, 384, 19–27.
Acknowledgements
The authors acknowledge Dr. Bimala Lama, the Department of Chemistry, and the Raman Microspectroscopy Laboratory at the University of Colorado Boulder for the use of NMR, UV-Vis-NIR, FL, and Raman spectroscopy instrumentation. The authors also acknowledge the Materials and Molecular Analysis Center at Colorado State University for the use of MALDI-TOF-MS instrumentation. This study was partly supported by the American Chemical Society Petroleum Research Fund Doctoral New Investigator grant (No. 59067-DNI7). Further support was provided by the College of Engineering and Applied Science at the University of Colorado Boulder. This work utilized resources from the University of Colorado Boulder Research Computing Group, which is supported by the National Science Foundation (awards ACI-1532235 and ACI-1532236), the University of Colorado Boulder, and Colorado State University.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2022_4145_MOESM1_ESM.pdf
All-hydrocarbon, all-conjugated cycloparaphenylene-polycyclic aromatic hydrocarbon host-guest complexes stabilized by CH−π interactions
Rights and permissions
About this article
Cite this article
Kwon, H., Bruns, C.J. All-hydrocarbon, all-conjugated cycloparaphenylene-polycyclic aromatic hydrocarbon host-guest complexes stabilized by CH−π interactions. Nano Res. 15, 5545–5555 (2022). https://doi.org/10.1007/s12274-022-4145-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4145-5