Abstract
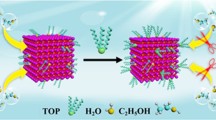
The poor stability of halide perovskite nanocrystals (NCs) has severely hindered future practical application. Herein, we proposed a facile and effective ligand modification route to synthesize stable CsPbBr3 nanocrystals by introducing a double-terminal ligand, namely 4,4′-Azobis(4-cyanovalericacid) (CA), to replace the conventional oleic acid (OA) ligand at room temperature. The as-synthesized CsPbBr3-CA not only possesses high photoluminescence quantum yield (72%) related to the reduced trap defects, but also shows significantly improved stability exposure to water, ethanol, light, and/or heat benefiting from the CA ligand anchored to NC surfaces tightly. The photoluminescence intensity of CsPbBr3-CA maintains about 80% and 75% of its initial emission intensity after immersed in water or ethanol for 360 min, respectively, whereas that of the CsPbBr3-OA was quenched completely within a few minutes. Moreover, an all-inorganic white light-emitting diode (LED) covered 126% National Television System Committee (NTSC) standard and 92% Rec.2020 standard was fabricated by combining the green CsPbBr3-CA and commercial red-emitting K2SiF6:Mn4+ (KSF) phosphors onto a blue LED chip. Thus, the presented work initiates the development of the room temperature preparation of high quality CsPbBr3 and shows prospect for next-generation displays.

Similar content being viewed by others
References
Huang, H.; Bodnarchuk, M. I.; Kershaw, S. V.; Kovalenko, M. V.; Rogach, A. L. Lead halide perovskite nanocrystals in the research spotlight: Stability and defect tolerance. ACS Energy Lett. 2017, 2, 2071–2083.
MacLaughlin, C. M. Opportunities and challenges in perovskite-based display technologies: A conversation with andrey rogach and Haibo Zeng. ACS Energy Lett. 2019, 4, 977–979.
Seth, S.; Ahmed, T.; De, A.; Samanta, A. Tackling the defects, stability, and photoluminescence of CsPbX3 perovskite nanocrystals. ACS Energy Lett. 2019, 4, 1610–1618.
Swarnkar, A.; Chulliyil, R.; Ravi, V. K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 perovskite nanocrystals: Luminescence beyond traditional quantum dots. Angew. Chem., Int. Ed. 2015, 54, 15424–15428.
Shamsi, J.; Urban, A. S.; Imran, M.; De Trizio, L.; Manna, L. Metal halide perovskite nanocrystals: Synthesis, post-synthesis modifications, and their optical properties. Chem. Rev. 2019, 119, 3296–3348.
Chouhan, L.; Ghimire, S.; Subrahmanyam, C.; Miyasaka, T.; Biju, V. Synthesis, optoelectronic properties and applications of halide perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885.
Yan, F.; Tan, S. T.; Li, X.; Demir, H. V. Light generation in lead halide perovskite nanocrystals: LEDs, color converters, lasers, and other applications. Small 2019, 15, 1902079.
Zheng, X. P.; Hou, Y.; Sun, H. T.; Mohammed, O. F.; Sargent, E. H.; Bakr, O. M. Reducing defects in halide perovskite nanocrystals for light-emitting applications. J. Phys. Chem. Lett. 2019, 10, 2629–2640.
Fang, Z. B.; Chen, W. J.; Shi, Y. L.; Zhao, J.; Chu, S. L.; Zhang, J.; Xiao, Z. G. Dual passivation of perovskite defects for light-emitting diodes with external quantum efficiency exceeding 20%. Adv. Funct. Mater. 2020, 30, 1909754.
Chiba, T.; Hayashi, Y.; Ebe, H.; Hoshi, K.; Sato, J.; Sato, S.; Pu, Y. J.; Ohisa, S.; Kido, J. Anion-exchange red perovskite quantum dots with ammonium iodine salts for highly efficient light-emitting devices. Nat. Photonics 2018, 12, 681–687.
Song, J. Z.; Li, J. H.; Xu, L. M.; Li, J. H.; Zhang, F. J.; Han, B. N.; Shan, Q. S.; Zeng, H. B. Room-temperature triple-ligand surface engineering synergistically boosts ink stability, recombination dynamics, and charge injection toward EQE-11.6% perovskite QLEDs. Adv. Mater. 2018, 30, 1800764.
Li, Y.; Wang, X. Y.; Xue, W. N.; Wang, W.; Zhu, W.; Zhao, L. J. Highly luminescent and stable CsPbBr3 perovskite quantum dots modified by phosphine ligands. Nano Res. 2019, 12, 785–789.
Voznyy, O. Black and stable: A path to all-inorganic halide perovskite solar cells. Joule 2018, 2, 1215–1216.
Chen, X.; Li, D. Y.; Pan, G. C.; Zhou, D. L.; Xu, W.; Zhu, J. Y.; Wang, H.; Chen, C.; Song, H. W. All-inorganic perovskite quantum dot/TiO2 inverse opal electrode platform: Stable and efficient photoelectrochemical sensing of dopamine under visible irradiation. Nanoscale 2018, 10, 10505–10513.
Sun, C.; Zhang, Y.; Ruan, C.; Yin, C. Y.; Wang, X. Y.; Wang, Y. D.; Yu, W. W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094.
Zhang, X. J.; Wang, H. C.; Tang, A. C.; Lin, S. Y.; Tong, H. C.; Chen, C. Y.; Lee, Y. C.; Tsai, T. L.; Liu, R. S. Robust and stable narrowband green emitter: An option for advanced wide-color-gamut backlight display. Chem. Mater. 2016, 28, 8493–8497.
He, Z. Q.; Zhang, C. C.; Dong, Y. J.; Wu, S. T. Emerging perovskite nanocrystals-enhanced solid-state lighting and liquid-crystal displays. Crystals 2019, 9, 59.
Nenon, D. P.; Pressler, K.; Kang, J.; Koscher, B. A.; Olshansky, J. H.; Osowiecki, W. T.; Koc, M. A.; Wang, L. W.; Alivisatos, A. P. Design principles for trap-free CsPbX3 Nanocrystals: Enumerating and eliminating surface halide vacancies with softer Lewis bases. J. Am. Chem. Soc. 2018, 140, 17760–17772.
Dai, J. F.; Xi, J.; Zu, Y. Q.; Li, L.; Xu, J.; Shi, Y. F.; Liu, X. Y.; Fan, Q. H.; Zhang, J. J.; Wang, S. P. et al. Surface mediated ligands addressing bottleneck of room-temperature synthesized inorganic perovskite nanocrystals toward efficient light-emitting diodes. Nano Energy 2020, 70, 104467.
Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A. F.; Masi, S.; Yoon, S. J.; Poyatos, M.; Julián-López, B.; Mora-Seró, I. Ligand & band gap engineering: Tailoring the protocol synthesis for achieving high-quality CsPbI3 quantum dots. Nanoscale 2020, 12, 14194–14203.
Shen, Z. H.; Zhao, S. L.; Song, D. D.; Xu, Z.; Qiao, B.; Song, P. J.; Bai, Q. Y.; Cao, J. Y.; Zhang, G. Q.; Swelm, W. Improving the quality and luminescence performance of all-inorganic perovskite nanomaterials for light-emitting devices by surface engineering. Small 2020, 16, 1907089
Ravi, V. K.; Saikia, S.; Yadav, S.; Nawale, V. V.; Nag, A. CsPbBr3/ZnS Core/Shell type nanocrystals for enhancing luminescence lifetime and water stability. ACS Energy Lett. 2020, 5, 1794–1796.
Pradhan, N. Tips and twists in making high photoluminescence quantum yield perovskite nanocrystals. ACS Energy Lett. 2019, 4, 1634–1638.
Wu, Y.; Wei, C. T.; Li, X. M.; Li, Y. L.; Qiu, S. C.; Shen, W.; Cai, B.; Sun, Z. G.; Yang, D. D.; Deng, Z. T. et al. In situ passivation of PbBr 4−6 octahedra toward blue luminescent CsPbBr3 nanoplatelets with near 100% absolute quantum yield. ACS Energy Lett. 2018, 3, 2030–2037.
Yang, D. D.; Li, X. M.; Wu, Y.; Wei, C. T.; Qin, Z. Y.; Zhang, C. F.; Sun, Z. G.; Li, Y. L.; Wang, Y.; Zeng, H. B. Surface halogen compensation for robust performance enhancements of CsPbX3 perovskite quantum dots. Adv. Opt. Mater. 2019, 7, 1900276.
Woo, J. Y.; Kim, Y.; Bae, J.; Kim, T. G.; Kim, J. W.; Lee, D. C.; Jeong, S. Highly stable cesium lead halide perovskite nanocrystals through in situ lead halide inorganic passivation. Chem. Mater. 2017, 29, 7088–7092.
Ahmed, T.; Seth, S.; Samanta, A. Boosting the photoluminescence of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals covering a wide wavelength range by postsynthetic treatment with tetrafluoroborate salts. Chem. Mater. 2018, 30, 3633–3637.
Koscher, B. A.; Swabeck, J. K.; Bronstein, N. D.; Alivisatos, A. P. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. J. Am. Chem. Soc. 2017, 139, 6566–6569.
Zhang, D. W.; Zhao, J.; Liu, Q. L.; Xia, Z. G. Synthesis and luminescence properties of CsPbX3@Uio-67 composites toward stable photoluminescence convertors. Inorg. Chem. 2019, 58, 1690–1696.
Xuan, T. T.; Huang, J. J.; Liu, H.; Lou, S. Q.; Cao, L. Y.; Gan, W. J.; Liu, R. S.; Wang, J. Super-hydrophobic cesium lead halide perovskite quantum dot-polymer composites with high stability and luminescent efficiency for wide color gamut white light-emitting diodes. Chem. Mater. 2019, 31, 1042–1047.
Tan, Y. S.; Zou, Y. T.; Wu, L. Z.; Huang, Q.; Yang, D.; Chen, M.; Ban, M. Y.; Wu, C.; Wu, T.; Bai, S. et al. Highly luminescent and stable perovskite nanocrystals with octylphosphonic acid as a ligand for efficient light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 3784–3792.
Krieg, F.; Ochsenbein, S. T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y. et al. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646.
Pan, J.; Shang, Y. Q.; Yin, J.; De Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A. M.; Hedhili, M. N.; Emwas, A. H. et al. Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes. J. Am. Chem. Soc. 2018, 140, 562–565.
Yang, D. D.; Li, X. M.; Zhou, W. H.; Zhang, S. L.; Meng, C. F.; Wu, Y.; Wang, Y.; Zeng, H. CsPbBr3 quantum dots 2.0: Benzenesulfonic acid equivalent ligand awakens complete purification. Adv. Mater. 2019, 31, 1900767.
Li, X. M.; Wu, Y.; Zhang, S. L.; Cai, B.; Gu, Y.; Song, J. Z.; Zeng, H. B. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445.
Dave, K.; Bao, Z.; Nakahara, S.; Ohara, K.; Masada, S.; Tahara, H.; Kanemitsu, Y.; Liu, R. S. Improvement in quantum yield by suppression of trions in room temperature synthesized CsPbBr3 perovskite quantum dots for backlight displays. Nanoscale 2020, 12, 3820–3826.
Li, F.; Liu, Y.; Wang, H. L.; Zhan, Q.; Liu, Q. L.; Xia, Z. G. Postsynthetic surface trap removal of CsPbX3 (X = Cl, Br, or I) quantum dots via a ZnX2/Hexane solution toward an enhanced luminescence quantum yield. Chem. Mater. 2018, 30, 8546–8554.
Pan, A. Z.; He, B.; Fan, X. Y.; Liu, Z. K.; Urban, J. J.; Alivisatos, A. P.; He, L.; Liu, Y. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: The role of organic acid, base, and cesium precursors. ACS Nano 2016, 10, 7943–7954.
Das, S.; De, A.; Samanta, A. Ambient condition Mg2+ doping producing highly luminescent green- and violet-emitting perovskite nanocrystals with reduced toxicity and enhanced stability. J. Phys. Chem. Lett. 2020, 11, 1178–1188.
Bao, Z.; Wang, W. G.; Tsai, H. Y.; Wang, H. C.; Chen, S. M.; Liu, R. S. Photo-/electro-luminescence enhancement of CsPbX3 (X = Cl, Br, or I) perovskite quantum dots via thiocyanate surface modification. J. Mater. Chem. C 2020, 8, 1065–1071.
Dai, J. F.; Xi, J.; Li, L.; Zhao, J. F.; Shi, Y. F.; Zhang, W. W.; Ran, C. X.; Jiao, B.; Hou, X.; Duan, X. H. et al. Charge transport between coupling colloidal perovskite quantum dots assisted by functional conjugated ligands. Angew. Chem., Int. Ed. 2018, 57, 5754–5758.
Tang, E. J.; Fu, C. Y.; Wang, S.; Dong, S. Y.; Zhao, F. Q.; Zhao, D. S. Graft polymerization of styrene monomer initiated by azobis(4-cyanovaleric acid) anchored on the surface of ZnO nanoparticles and its PVC composite film. Powder Technol. 2012, 218, 5–10.
Wang, S. X.; Wang, Y.; Zhang, Y.; Zhang, X. T.; Shen, X Y.; Zhuang, X. W.; Lu, P.; Yu, W. W.; Kershaw, S. V.; Rogach, A. L. Cesium lead chloride/bromide perovskite quantum dots with strong blue emission realized via a nitrate-induced selective surface defect elimination process. J. Phys. Chem. Lett. 2019, 10, 90–96.
Shi, Y. F.; Wu, W.; Dong, H.; Li, G. R.; Xi, K.; Divitini, G.; Ran, C. X.; Yuan, F.; Zhang, M.; Jiao, B. et al. A strategy for architecture design of crystalline perovskite light-emitting diodes with high performance. Adv. Mater. 2018, 30, 1800251.
Luo, B. B.; Pu, Y. C.; Lindley, S. A.; Yang, Y.; Lu, L. Q.; Li, Y.; Li, X. M.; Zhang, J. Z. Organolead halide perovskite nanocrystals: Branched capping ligands control crystal size and stability. Angew. Chem., Int. Ed. 2016, 55, 8864–8868.
Xuan, T. T.; Yang, X. F.; Lou, S. Q.; Huang, J. Q.; Liu, Y.; Yu, J. B.; Li, H. L.; Wong, K. L.; Wang, C. X.; Wang, J. Highly stable CsPbBr3 quantum dots coated with alkyl phosphate for white light-emitting diodes. Nanoscale 2017, 9, 15286–15290.
Acknowldgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 61775060, 61275100, 61761136006, 61790583, and 61874043).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2021_3283_MOESM1_ESM.pdf
Room temperature preparation of highly stable cesium lead halide perovskite nanocrystals by ligand modification for white light-emitting diodes
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, G., She, C. et al. Room temperature preparation of highly stable cesium lead halide perovskite nanocrystals by ligand modification for white light-emitting diodes. Nano Res. 14, 2770–2775 (2021). https://doi.org/10.1007/s12274-021-3283-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3283-5