Abstract
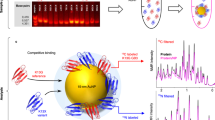
The interaction of nanoparticles with proteins is extremely complex, important for understanding the biological properties of nanomaterials, but is very poorly understood. We have employed a combinatorial library of surface modified gold nanoparticles to interrogate the relationships between the nanoparticle surface chemistry and the specific and nonspecific binding to a common, important, and representative enzyme, acetylcholinesterase (AChE). We also used Bayesian neural networks to generate robust quantitative structure-property relationship (QSPR) models relating the nanoparticle surface to the AChE binding that also provided significant understanding into the molecular basis for these interactions. The results illustrate the insights that result from a synergistic blending of experimental combinatorial synthesis and biological testing of nanoparticles with quantitative computational methods and molecular modeling.

Similar content being viewed by others
References
Epa, V. C.; Winkler, D. A.; Tran, L.; Fadeel, B.; Pietroiusti, A.; Shvedova, A. A. Computational approaches. In Adverse effects of engineered nanomaterials: Exposure, toxicology, and impact on human health; Fadeel, B.; Pietroiusti, A.; Shvedova, A. A., Eds.; Academic Press: London, 2012; pp 85–96.
Maynard, A.; Rejeski, D. Too small to overlook. Nature 2009, 460, 174–174.
Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627.
Hartig, W.; Kacza, J.; Paulke, B. R.; Grosche, J.; Bauer, U.; Hoffmann, A.; Elsinghorst, P. W.; Gutschow, M. In vivo labelling of hippocampal beta-amyloid in triple-transgenic mice with a fluorescent acetylcholinesterase inhibitor released from nanoparticles. Euro. J. Neurosci. 2010, 31, 99–109.
Winkler, D. A.; Mombelli, E.; Pietroiusti, A.; Tran, L.; Worth, A.; Fadeel, B.; McCall, M. J. Applying quantitative structure-activity relationship approaches to nanotoxicology: Current status and future potential. Toxicology 2013, 313, 15–23.
Zhang, B.; Xing, Y. H.; Li, Z. W.; Zhou, H. Y.; Mu, Q. X.; Yan, B. Functionalized carbon nanotubes specifically bind to α-chymotrypsin’s catalytic site and regulate its enzymatic function. Nano Lett. 2009, 9, 2280–2284.
Zhou, H. Y.; Mu, Q. X.; Gao, N. N.; Liu, A. F.; Xing, Y. H.; Gao, S. L.; Zhang, Q.; Qu, G. B.; Chen, Y. Y.; Liu, G. et al. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity, and immune response. Nano Lett. 2008, 8, 859–865.
Weissleder, R.; Kelly, K.; Sun, E. Y.; Shtatland, T.; Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005, 23, 1418–1423.
You, C. C.; De, M.; Han, G.; Rotello, V. M. Tunable inhibition and denaturation of α-chymotrypsin with amino acid-functionalized gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 12873–12881.
Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K. A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055.
Lesniak, A.; Fenaroli, F.; Monopoli, M. R.; Aberg, C.; Dawson, K. A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857.
Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K. A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270.
Epa, V. C.; Burden, F. R.; Tassa, C.; Weissleder, R.; Shaw, S.; Winkler, D. A. Modeling biological activities of nanoparticles. Nano Lett. 2012, 12, 5808–5812.
Yan, Y.; Bjoernmalm, M.; Caruso, F. Particle carriers for combating multidrug-resistant cancer. ACS Nano 2013, 7, 9512–9517.
Calvaresi, M.; Arnesano, F.; Bonacchi, S.; Bottoni, A.; Calò, V.; Conte, S.; Falini, G.; Fermani, S.; Losacco, M.; Montalti, M. et al. C60@lysozyme: Direct observation by nuclear magnetic resonance of a 1:1 fullerene protein adduct. ACS Nano 2014, 8, 1871–1877.
Rotundo, R. L. Expression and localization of acetylcholine-esterase at the neuromuscular junction. J. Neurocytol. 2003, 32, 743–766.
Giacobini, E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr Psych. 2003, 18, S1–S5.
Perry, E. K.; Perry, R. H.; Blessed, G.; Tomlinson, B. E. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropath. Appl. Neuro. 1978, 4, 273–277.
Perry, E. K.; Tomlinson, B. E.; Blessed, G.; Bergmann, K.; Gibson, P. H.; Perry, R. H. Correlation of cholinergic abnormalities with senile plaques and mental test-scores in senile dementia. Brit. Med. J. 1978, 2, 1457–1459.
Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C. et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. New Engl. J. Med. 2012, 366, 893–903.
Pastorin, G.; Marchesan, S.; Hoebeke, J.; Da Ros, T.; Ehret-Sabatier, L.; Briand, J. P.; Prato, M.; Bianco, A. Design and activity of cationic fullerene derivatives as inhibitors of acetylcholinesterase. Org. Biomol. Chem. 2006, 4, 2556–2562.
Aggarwal, P.; Hall, J. B.; McLeland, C. B.; Dobrovolskaia, M. A.; McNeil, S. E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliver. Rev. 2009, 61, 428–437.
Adikrisna, R.; Tanaka, S.; Muramatsu, S.; Aihara, A.; Ban, D.; Ochiai, T.; Irie, T.; Kudo, A.; Nakamura, N.; Yamaoka, S. et al. Identification of pancreatic cancer stem cells and selective toxicity of chemotherapeutic agents. Gastroenterology. 2012, 143, 234–245.
Mu, Q. X.; Liu, W.; Xing, Y. H.; Zhou, H. Y.; Li, Z. W.; Zhang, Y.; Ji, L. H.; Wang, F.; Si, Z. K.; Zhang, B. et al. Protein binding by functionalized multiwalled carbon nanotubes is governed by the surface chemistry of both parties and the nanotube diameter. J. Phys. Chem. C 2008, 112, 3300–3307.
Zhou, H. Y.; Jiao, P. F.; Yang, L.; Li, X.; Yan, B. Enhancing cell recognition by scrutinizing cell surfaces with a nanoparticle array. J. Am. Chem. Soc. 2011, 133, 680–682.
Zhou, H. Y.; Li, X.; Lemoff, A.; Zhang, B.; Yan, B. Structural confirmation and quantification of individual ligands from the surface of multi-functionalized gold nanoparticles. Analyst 2010, 135, 1210–1213.
Chen, Y.; Barkley, M. D. Toward understanding tryptophan fluorescence in proteins. Biochem. 1998, 37, 9976–9982.
Crammer, J.; Neuberger, A. The state of tyrosine in egg albumin and in insulin as determined by spectrophotometric titration. Biochem. J. 1943, 37, 302–310.
Teale, F. W. J.; Weber, G. Ultraviolet fluorescence of the aromatic amino acids. Biochem. J. 1957, 65, 476–482.
Epa, V. C.; Yang, J.; Mei, Y.; Hook, A. L.; Langer, R.; Anderson, D. G.; Davies, M. C.; Alexander, M. R.; Winkler, D. A. Modelling human embryoid body cell adhesion to a combinatorial library of polymer surfaces. J. Mater. Chem. 2012, 22, 20902–20906.
Burden, F. R.; Winkler, D. A. Optimal sparse descriptor selection for QSAR using Bayesian methods. QSAR Comb. Sci. 2009, 28, 645–653.
Burden, F. R.; Winkler, D. A. Robust QSAR models using Bayesian regularized neural networks. J. Med. Chem. 1999, 42, 3183–3187.
Burden, F. R.; Winkler, D. A. An optimal self-pruning neural network and nonlinear descriptor selection in QSAR. QSAR Comb. Sci. 2009, 28, 1092–1097.
Salahinejad, M.; Le, T. C.; Winkler, D. A. Aqueous solubility prediction: Do crystal lattice interactions help? Mol. Pharmaceut. 2013, 10, 2757–2766.
Salahinejad, M.; Le, T. C.; Winkler, D. A. Capturing the crystal: Prediction of enthalpy of sublimation, crystal lattice energy, and melting points of organic compounds. J. Chem. Inf. Model. 2013, 53, 223–229.
Cheung, J.; Gary, E. N.; Shiomi, K.; Rosenberry, T. L. Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096.
Ellman, G. L.; Courtney, K. D.; Andres, V.; Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95.
Eftink, M. R.; Zajicek, J. L.; Ghiron, C. A. Hydrophobic quencher of protein fluorescence-2,2,2-trichloroethanol. Biochim. Biophys. Acta 1977, 491, 473–481.
Mauri, A.; Consonni, V.; Pavan, M.; Todeschini, R. DRAGON software: An easy approach to molecular descriptor calculations. MATCHCommun. Math. Co. 2006, 56, 237–248.
Le, T.; Epa, V. C.; Burden, F. R.; Winkler, D. A. Quantitative structure-property relationship modeling of diverse materials properties. Chem. Rev. 2012, 112, 2889–2919.
Hagan, M. T.; Menhaj, M. Training feed forward networks with the Marquardt algorithm. IEEE Trans. Neural Netw. 1994, 5, 989–993.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Y., Winkler, D.A., Epa, V.C. et al. Probing enzyme-nanoparticle interactions using combinatorial gold nanoparticle libraries. Nano Res. 8, 1293–1308 (2015). https://doi.org/10.1007/s12274-014-0618-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0618-5