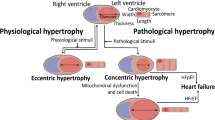
Abstract
Rehabilitation exercise is a crucial non-pharmacological intervention for the secondary prevention and treatment of cardiovascular diseases, effectively ameliorating cardiac remodeling in patients. Exercise training can mitigate cardiomyocyte apoptosis, reduce extracellular matrix deposition and fibrosis, promote angiogenesis, and regulate inflammatory response to improve cardiac remodeling. This article presents a comprehensive review of recent research progress, summarizing the pivotal role and underlying mechanism of rehabilitation exercise in improving cardiac remodeling and providing valuable insights for devising effective rehabilitation treatment programs.

Graphical Abstract

Similar content being viewed by others
References
Jegier A, et al. Comprehensive cardiac rehabilitation as the keystone in the secondary prevention of cardiovascular disease. Kardiol Pol. 2021;79(7–8):901–16.
Knuuti J, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Fang J, et al. Use of outpatient cardiac rehabilitation among heart attack survivors - 20 states and the District of Columbia, 2013 and four states, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(33):869–73.
Gabrys L, Schmidt C. Prescription and utilization of sports therapy programs following cardiac rehabilitation 2006–2013. Rehabilitation (Stuttg). 2020;59(1):42–7.
You-gen ZHOU, et al. Influence of cognitive behavioral therapy on psychology and compliance to exercise prescription in CHD patients. Chinese Journal of Cardiovascular Rehabilitation Medicine. 2018;27(4):369–72.
Baman JR, Sekhon S, Maganti K. Cardiac rehabilitation. JAMA. 2021;326(4):366.
Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11(11):e005263.
Zhou MC, Hong Y. Updated essentials of scientific exercise and training in the 6th edition of the guidelines for cardiac rehabilitation programs by American Association of Cardiovascular and Pulmonary Rehabilitation [J]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2021;29(6):1–6.
Cornelis J, et al. Comparing exercise training modalities in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2016;221:867–76.
Arnett DK, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–646.
Feito Y, et al. High-Intensity Functional Training (HIFT): Definition and research implications for improved fitness. Sports (Basel). 2018;6(3).
Ribeiro P, et al. High-intensity interval training in patients with coronary heart disease: prescription models and perspectives. Ann Phys Rehabil Med. 2017;60(1):50–7.
Eser P. et al. Short- and long-term effects of high-intensity interval training vs. moderate-intensity continuous training on left ventricular remodeling in patients early after ST-segment elevation myocardial infarction-The HIIT-EARLY randomized controlled trial. Front Cardiovasc Med. 2022;9:869501.
Dayan A, et al. Swimming exercise training prior to acute myocardial infarction attenuates left ventricular remodeling and improves left ventricular function in rats. Ann Clin Lab Sci. 2005;35(1):73–8.
Zhao S, et al. Effect of Tai Chi on cardiac function in patients with myocardial infarction: a protocol for a randomized controlled trial. Medicine (Baltimore). 2021;100(42):e27446.
Mao S. et al. Baduanjin Exercise prevents post-myocardial infarction left ventricular remodeling (BE-PREMIER trial): Design and rationale of a pragmatic randomized controlled trial. Cardiovasc Drugs Ther. 2016;30(3):315–22.
Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol. 2012;302(1):H10-23.
Wang B, et al. Effect of high-intensity interval training on cardiac structure and function in rats with acute myocardial infarct. Biomed Pharmacother. 2020;131:110690.
Souza LM, et al. Effects of late aerobic exercise on cardiac remodeling of rats with small-sized myocardial infarction. Arq Bras Cardiol. 2021;116(4):784–92.
Liao Z, et al. Early moderate exercise benefits myocardial infarction healing via improvement of inflammation and ventricular remodelling in rats. J Cell Mol Med. 2019;23(12):8328–42.
Guizoni DM, et al. Effects of late exercise on cardiac remodeling and myocardial calcium handling proteins in rats with moderate and large size myocardial infarction. Int J Cardiol. 2016;221:406–12.
Cai M, Wang L, Ren YL. Effect of exercise training on left ventricular remodeling in patients with myocardial infarction and possible mechanisms. World J Clin Cases. 2021;9(22):6308–18.
Marcin T, et al. Effect of self-tailored high-intensity interval training versus moderate-intensity continuous exercise on cardiorespiratory fitness after myocardial infarction: a randomised controlled trial. Ann Phys Rehabil Med. 2022;65(1):101490.
Trachsel LD, et al. The impact of high-intensity interval training on ventricular remodeling in patients with a recent acute myocardial infarction-a randomized training intervention pilot study. Clin Cardiol. 2019;42(12):1222–31.
Guo Y, et al. Cardiomyocyte homeodomain-interacting protein kinase 2 maintains basal cardiac function via extracellular signal-regulated kinase signaling. Circulation. 2019;140(22):1820–33.
Zhou Q, et al. Exercise downregulates HIPK2 and HIPK2 inhibition protects against myocardial infarction. EBioMedicine. 2021;74: 103713.
Shi J, et al. miR-17-3p Contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics. 2017;7(3):664–76.
Yu Y, et al. Exercise-generated β-aminoisobutyric acid (BAIBA) reduces cardiomyocyte metabolic stress and apoptosis caused by mitochondrial dysfunction through the miR-208b/AMPK pathway. Front Cardiovasc Med. 2022;9:803510.
Wu X, et al. ADAR2 increases in exercised heart and protects against myocardial infarction and doxorubicin-induced cardiotoxicity. Mol Ther. 2022;30(1):400–14.
Gao R, et al. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation. 2021;144(4):303–17.
Peixoto TC, et al. Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: a randomized controlled trial. Can J Cardiol. 2015;31(3):308–13.
Ghardashi AA, et al. Targeting necroptotic cell death pathway by high-intensity interval training (HIIT) decreases development of post-ischemic adverse remodelling after myocardial ischemia/reperfusion injury. J Cell Commun Signal. 2019;13(2):255–67.
Bo W, et al. The roles of FGF21 and ALCAT1 in aerobic exercise-induced cardioprotection of postmyocardial infarction mice. Oxid Med Cell Longev. 2021;2021:8996482.
Ma Y, et al. Exercise training alleviates cardiac fibrosis through increasing fibroblast growth factor 21 and regulating TGF-β1-Smad2/3-MMP2/9 signaling in mice with myocardial infarction. Int J Mol Sci. 2021;22(22).
Jia D, et al. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J Cell Physiol. 2019;234(12):23705–18.
Qu X, et al. MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep. 2017;7:42657.
Zhang JC, et al. Effect of lncRNA GAS5 on rats with acute myocardial infarction through regulating miR-21. Eur Rev Med Pharmacol Sci. 2019;23(19):8573–9.
Farsangi SJ, et al. Modulation of the expression of long non-coding RNAs H19, GAS5, and MIAT by endurance exercise in the hearts of rats with myocardial infarction. Cardiovasc Toxicol. 2021;21(2):162–8.
Song W, et al. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats. J Cell Mol Med. 2020;24(22):12970–9.
Xi Y, et al. Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A-Smad2/3 in rats following myocardial infarction. J Sport Health Sci. 2021;10(5):594–03.
Cai MX, et al. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 2016;149:1–9.
Shi X, Luo X, Xu X. Dimethylarginine dimethylaminohydrolase-1 contributes to exercise-induced cardiac angiogenesis in mice. Biosci Trends. 2020;14(2):115–22.
Xia WH, et al. Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell. 2012;11(1):111–9.
Jayo-Montoya JA, et al. Chronotropic responses to exercise and recovery in myocardial infarction patients taking β-blockers following aerobic high-intensity interval training: an interfarct study. J Cardiopulm Rehabil Prev. 2022;42(1):22–7.
Khadanga S, et al. Optimizing training response for women in cardiac rehabilitation: a randomized clinical trial. JAMA Cardiol. 2022;7(2):215–8.
Yakut H, et al. Effect of home-based high-intensity interval training versus moderate-intensity continuous training in patients with myocardial infarction: a randomized controlled trial. Ir J Med Sci. 2022;191(6):2539–48.
Dor-Haim H, et al. Intermittent aerobic-resistance interval training versus continues aerobic training: improvement in cardiac electrophysiologic and anthropometric measures in male patients post myocadiac infarction, a randomized control trial. PLoS ONE. 2022;17(5):e0267888.
Eser P, et al. Acute and chronic effects of high-intensity interval and moderate-intensity continuous exercise on heart rate and its variability after recent myocardial infarction: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65(1):101444.
Kollet DP, et al. Aerobic exercise, but not isometric handgrip exercise, improves endothelial function and arterial stiffness in patients with myocardial infarction undergoing coronary intervention: a randomized pilot study. BMC Cardiovasc Disord. 2021;21(1):101.
Jiang M, et al. Effect analysis of kinetic energy progressive exercise in patients with acute myocardial infarction after percutaneous coronary intervention: a randomized trial. Ann Palliat Med. 2021;10(7):7823–31.
Grabara M, Nowak Z, Nowak A. Effects of Hatha yoga on cardiac hemodynamic parameters and physical capacity in cardiac rehabilitation patients. J Cardiopulm Rehabil Prev. 2020;40(4):263–7.
McGREGOR G, et al. Reverse left ventricular remodeling: effect of cardiac rehabilitation exercise training in myocardial infarction patients with preserved ejection fraction. Eur J Phys Rehabil Med. 2016;52(3):370–8.
Giallauria F, et al. Effects of exercise-based cardiac rehabilitation on high mobility group box-1 levels after acute myocardial infarction: rationale and design. J Cardiovasc Med. 2009;10(8):659–63.
Kubo N, et al. Exercise at ventilatory threshold aggravates left ventricular remodeling in patients with extensive anterior acute myocardial infarction. Am Heart J. 2004;147(1):113–20.
Chambers J. Aortic stenosis. BMJ. 2005;330(7495):801–2.
Yap SC, et al. Aortic stenosis at young adult age. Expert Rev Cardiovasc Ther. 2005;3(6):1087–98.
Zeppilli P, et al. Bicuspid aortic valve: an innocent finding or a potentially life-threatening anomaly whose complications may be elicited by sports activity? J Cardiovasc Med (Hagerstown). 2006;7(4):282–7.
Scharhag J, et al. Bicuspid aortic valve: evaluation of the ability to participate in competitive sports: case reports of two soccer players. Clin Res Cardiol. 2006;95(4):228–34.
Schultz RL, et al. Effects of excessive long-term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension. 2007;50(2):410–6.
[Guidelines for cardiovascular rehabilitation and secondary prevention in China 2018 simplified edition]. Zhonghua Nei Ke Za Zhi. 2018;57(11):802–10.
Ravassa S, et al. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: a population-based study. Int J Cardiol. 2015;185:177–85.
Humeres C, Frangogiannis NG. fibroblasts in the infarcted, remodeling, and failing heart. JACC Basic Transl Sci. 2019;4(3):449–67.
Lim SL, et al. Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur Heart J. 2015;36(31):2050–60.
Huang H, Huang W. Regulation of endothelial progenitor cell functions in ischemic heart disease: New therapeutic targets for cardiac remodeling and repair. Front Cardiovasc Med. 2022;9:896782.
Su SA, et al. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget. 2017;8(15):25700-25712.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407.
Ponnusamy M, et al. Long Noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation. 2019;139(23):2668–84.
Liu X, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21(4):584–95.
Yang J, et al. Excessive treadmill training produces different cardiac-related microRNA profiles in the left and right ventricles in mice. Int J Sports Med. 2022;43(3):219–29.
Mathiyalagan P, et al. FTO-dependent N(6)-methyladenosine regulates cardiac function during remodeling and repair. Circulation. 2019;139(4):518–32.
Zhang T, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22(2):175–82.
Zhang X, et al. Fibronectin type III domain-containing 5 in cardiovascular and metabolic diseases: a promising biomarker and therapeutic target. Acta Pharmacol Sin. 2021;42(9):1390–400.
Hassaan PS, et al. Irisin vs. Treadmill exercise in post myocardial infarction cardiac rehabilitation in rats. ARCH MED RES. 2019;50(2):44–54.
Lee SE, et al. Three-dimensional cardiomyocytes structure revealed by diffusion tensor imaging and its validation using a tissue-clearing technique. Sci Rep. 2018;8(1):6640.
Eder RA, et al. Exercise-induced CITED4 expression is necessary for regional remodeling of cardiac microstructural tissue helicity. Commun Biol. 2022;5(1):656.
Boström P, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143(7):1072–83.
Bezzerides VJ, et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1(9).
Varga I, et al. The non-cardiomyocyte cells of the heart. Their possible roles in exercise-induced cardiac regeneration and remodeling. Adv Exp Med Biol. 2017;999:117–36.
Davis J, et al. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23(4):705–15.
Fernandes T, et al. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol. 2015;309(4):H543–52.
Opstad TB, et al. MMP-9 and its regulators TIMP-1 and EMMPRIN in patients with acute ST-elevation myocardial infarction: a NORDISTEMI substudy. Cardiology. 2018;139(1):17–24.
Brianezi L, et al. Effects of Physical training on the myocardium of oxariectomized LDLr knockout mice: MMP 2/9, collagen I/III, inflammation and oxidative stress. Arq Bras Cardiol. 2020;114(1):100–5.
Lighthouse JK, et al. Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2019;4(1).
Cai Y, et al. Aerobic exercise prevents insulin resistance through the regulation of miR-492/resistin axis in aortic endothelium. J Cardiovasc Transl Res. 2018;11(6):450–8.
Donghui T, et al. Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Microvasc Res. 2019;123:86–91.
Ouchi N, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283(47):32802–11.
Xi Y, et al. Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A-Smad2/3 in rats following myocardial infarction. J Sport Health Sci. 2021;10(5):594–603.
Pourheydar B, et al. Exercise improves aging-related decreased angiogenesis through modulating VEGF-A, TSP-1 and p-NF-Ƙb protein levels in myocardiocytes. J Cardiovasc Thorac Res. 2020;12(2):129–35.
Chen J, et al. The impact of cardiomotor rehabilitation on endothelial function in elderly patients with chronic heart failure. BMC cardiovasc disord. 2021;21(1):524.
Li WD, et al. LncRNA WTAPP1 promotes migration and angiogenesis of endothelial progenitor cells via MMP1 through MicroRNA 3120 and Akt/PI3K/autophagy pathways. Stem cells. 2018;36(12):1863–74.
Soori R, et al. Exercise attenuates myocardial fibrosis and increases angiogenesis-related molecules in the myocardium of aged rats. Arch Physiol Biochem. 2022;128(1):1–6.
Jin K, et al. Single-cell RNA sequencing reveals the temporal diversity and dynamics of cardiac immunity after myocardial infarction. Small Methods. 2022;6(3):e2100752.
Zhang QL, et al. GRGM-13 comprising 13 plant and animal products, inhibited oxidative stress induced apoptosis in retinal ganglion cells by inhibiting P2RX7/p38 MAPK signaling pathway. Biomed Pharmacother. 2018;101:494–500.
Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122(12):1722–40.
Afonina IS, et al. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–9.
Stachon P, et al. P2X(7) deficiency blocks lesional inflammasome activity and ameliorates atherosclerosis in mice. Circulation. 2017;135(25):2524–33.
Chen X, et al. Aerobic exercise ameliorates myocardial inflammation, fibrosis and apoptosis in high-fat-diet rats by inhibiting P2X7 purinergic receptors. Front Physiol. 2019;10:1286.
Peake JM, et al. Recovery of the immune system after exercise. J Appl Physiol (1985). 2017;122(5):1077–87.
Femminò S, et al. Extracellular vesicles and cardiovascular system: biomarkers and cardioprotective effectors. Vascul Pharmacol. 2020;135: 106790.
Bei Y, et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol. 2017;112(4):38.
Yin A, et al. Exercise-derived peptide protects against pathological cardiac remodeling. EBioMedicine. 2022;82: 104164.
Funding
This work was supported by the China Postdoctoral Science Foundation (71th Batch-2022M711321), the Jining Medical University Research Fund for Academician Lin He New Medicine (JYHL2022), the Shandong Province Key Project of TCM Science and Technology (Z-2022081), and the Key research and development plan in Jining City (2022YXNS003).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The first draft of the manuscript was written by Haizhu Gao. Writing is supervised and guided by Xueying Chen and Lijun Gan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors report no potential conflicts of interest relevant to this study.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, H., Li, Z., Gan, L. et al. The Role and Potential Mechanisms of Rehabilitation Exercise Improving Cardiac Remodeling. J. of Cardiovasc. Trans. Res. (2024). https://doi.org/10.1007/s12265-024-10498-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-024-10498-7