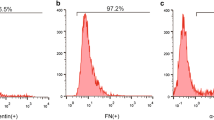
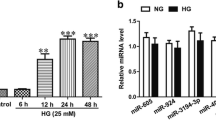
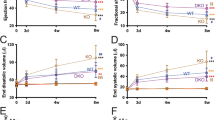
Abstract
It is widely accepted that miRNAs play an important role in the pathogenesis of myocardial fibrosis. This study aimed to identify a new pathway of miR-212-5p in the activation of human cardiac fibroblasts (HCFs) induced by oxygen–glucose deprivation (OGD). First, we found that KLF4 protein was markedly decreased in OGD-induced HCFs. Then, bioinformatics analysis and verification experiments were used to identify the existence of an interaction of KLF4 with miR-212-5p. Functional experiments indicated that OGD significantly upregulated the expression of hypoxia inducible factor-1 alpha (HIF-1α) in HCFs, which positively regulated miR-212-5p transcription by binding to its promoter. MiR-212-5p inhibited the expression of Krüppel-like factor 4 (KLF4) protein by binding to the 3’ untranslated coding regions (UTRs) of KLF4 mRNA. Inhibition of miR-212-5p effectively inhibited the activation of OGD-induced HCFs by upregulating KLF4 expression and inhibited cardiac fibrosis in vivo and in vitro.
Graphical Abstract







Similar content being viewed by others
Abbreviations
- OGD:
-
Oxygen and glucose deprivation
- HIF-1α:
-
Hypoxia inducible factor-1 alpha
- KLF4:
-
Krüppel-like factor 4
- CFs:
-
Cardiac fibroblasts
- Lv:
-
Lentivirus
- LV:
-
Left ventricle
- IVS:
-
Interventricular septal thickness
- LVPW:
-
Left ventricle posterior wall thickness
References
Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. https://doi.org/10.1016/S0140-6736(16)30677-8.
Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–65. https://doi.org/10.1056/NEJMoa0908610.
Park S, Nguyen NB, Pezhouman A, Ardehali R. Cardiac fibrosis: potential therapeutic targets. Transl Res. 2019;209:121–37. https://doi.org/10.1016/j.trsl.2019.03.001.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65. https://doi.org/10.1038/nrcardio.2014.28.
Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–75. https://doi.org/10.1172/JCI31044.
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–16. https://doi.org/10.2353/ajpath.2007.070112.
Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract. 2017;133:124–30. https://doi.org/10.1016/j.diabres.2017.08.018.
Pichler M, Rainer PP, Schauer S, Hoefler G. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol. 2012;59(11):1008–16. https://doi.org/10.1016/j.jacc.2011.11.036.
Wu C, Liu B, Wang R, Li G. The regulation mechanisms and clinical application of microRNAs in myocardial infarction: a review of the recent 5 years. Front Cardiovasc Med. 2021;8:809580. https://doi.org/10.3389/fcvm.2021.809580.
Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. https://doi.org/10.1038/ncomms2090.
Gupta SK, Garg A, Avramopoulos P, Engelhardt S, Streckfuss-Bomeke K, Batkai S, et al. miR-212/132 cluster modulation prevents doxorubicin-mediated atrophy and cardiotoxicity. Mol Ther. 2019;27(1):17–28. https://doi.org/10.1016/j.ymthe.2018.11.004.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Lu S, Jolly AJ, Strand KA, Dubner AM, Mutryn MF, Moulton KS, et al. Smooth muscle-derived progenitor cell myofibroblast differentiation through KLF4 downregulation promotes arterial remodeling and fibrosis. JCI Insight. 2020;5(23). https://doi.org/10.1172/jci.insight.139445.
Xu Y, Luo Y, Liang C, Zhang T. LncRNA-Mhrt regulates cardiac hypertrophy by modulating the miR-145a-5p/KLF4/myocardin axis. J Mol Cell Cardiol. 2020;139:47–61. https://doi.org/10.1016/j.yjmcc.2019.12.013.
Farina FM, Hall IF, Serio S, Zani S, Climent M, Salvarani N, et al. miR-128-3p is a novel regulator of vascular smooth muscle cell phenotypic switch and vascular diseases. Circ Res. 2020;126(12):e120–35. https://doi.org/10.1161/CIRCRESAHA.120.316489.
Song HF, He S, Li SH, Wu J, Yin W, Shao Z, et al. Knock-out of microRNA 145 impairs cardiac fibroblast function and wound healing post-myocardial infarction. J Cell Mol Med. 2020;24(16):9409–19. https://doi.org/10.1111/jcmm.15597.
Kluiver J, Gibcus JH, Hettinga C, Adema A, Richter MK, Halsema N, et al. Rapid generation of microRNA sponges for microRNA inhibition. PLoS One. 2012;7(1):e29275. https://doi.org/10.1371/journal.pone.0029275.
Gonzalez A, Schelbert EB, Diez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71(15):1696–706. https://doi.org/10.1016/j.jacc.2018.02.021.
Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15. https://doi.org/10.1186/1755-1536-5-15.
Ma Y, Halade GV, Lindsey ML. Extracellular matrix and fibroblast communication following myocardial infarction. J Cardiovasc Transl Res. 2012;5(6):848–57. https://doi.org/10.1007/s12265-012-9398-z.
Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110(14):5588–93. https://doi.org/10.1073/pnas.1301019110.
Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–55. https://doi.org/10.1016/j.ajpath.2012.02.004.
Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63(22):2335–45. https://doi.org/10.1016/j.jacc.2014.02.555.
da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118(15):1567–76. https://doi.org/10.1161/CIRCULATIONAHA.108.769984.
Mayer SC, Gilsbach R, Preissl S, Monroy Ordonez EB, Schnick T, Beetz N, et al. Adrenergic repression of the epigenetic reader MeCP2 facilitates cardiac adaptation in chronic heart failure. Circ Res. 2015;117(7):622–33. https://doi.org/10.1161/CIRCRESAHA.115.306721.
Sarkozy M, Gaspar R, Zvara A, Kiscsatari L, Varga Z, Kovari B, et al. Selective heart irradiation induces cardiac overexpression of the pro-hypertrophic miR-212. Front Oncol. 2019;9:598. https://doi.org/10.3389/fonc.2019.00598.
Wu Y, Peng W, Fang M, Wu M, Wu M. MSCs-derived extracellular vesicles carrying miR-212-5p alleviate myocardial infarction-induced cardiac fibrosis via NLRC5/VEGF/TGF-beta1/SMAD axis. J Cardiovasc Transl Res. 2022;15(2):302–16. https://doi.org/10.1007/s12265-021-10156-2.
Parisi Q, Biondi-Zoccai GG, Abbate A, Santini D, Vasaturo F, Scarpa S, et al. Hypoxia inducible factor-1 expression mediates myocardial response to ischemia late after acute myocardial infarction. Int J Cardiol. 2005;99(2):337–9. https://doi.org/10.1016/j.ijcard.2003.11.038.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Zhang Y, Wang Y, Liu Y, Wang N, Qi Y, Du J. Kruppel-like factor 4 transcriptionally regulates TGF-beta1 and contributes to cardiac myofibroblast differentiation. PLoS One. 2013;8(4):e63424. https://doi.org/10.1371/journal.pone.0063424.
Funding
This study was supported by National Natural Science Foundation of China (Grant numbers 81570406, 81500219, and 81870330) and Central University Basic Science Foundation of China (Grant number 1191329724).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(TIF 2.05 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Li, C., Zheng, T. et al. Cardiac Fibroblast Activation Induced by Oxygen–Glucose Deprivation Depends on the HIF-1α/miR-212-5p/KLF4 Pathway. J. of Cardiovasc. Trans. Res. 16, 778–792 (2023). https://doi.org/10.1007/s12265-023-10360-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10360-2