Abstract
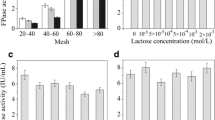
The biodegradation of straw by cellulose-degrading strains plays an important role in the disposal of agricultural waste. In this study, a Penicillium sp. strain JiTF01 that could degrade cellulose was isolated at the low temperature (10°C), and its fermentation conditions were optimized by response surface method. Strain JiTF01 was applied to degrade rice straw at 10°C. It was found that the straw degradation ratio could reach to 46.53% on the 25th day of culture with the enzyme activity of 44.30 U/mL under optimized fermentation conditions. In addition, given that strain JiTF01 showed excellent acid resistance and the cellulase produced by strain JiTF01 exhibited higher enzymatic activity and stability under acidic conditions, the uninterrupted integration process of acid pretreatment and acid fermentation of straw was then performed. The results showed that the degradation ratio of 53.61% was observed after 25 days of the fermentation at pH 3, which increased by 7.08% than that under optimized conditions. Besides, the effect of straw fermentation broth on the growth of rice seed was also investigated, and the 10−5 and 10−6 diluted fermentation broth was found that could significantly promote the growth of rice seedlings and enhance the germination rate of rice seeds under the salt stress of 100 and 200 mmol/L. The conclusions found in this article might have important implications for straw recycling in cold regions.
Similar content being viewed by others
References
Zheng, G., T. Yin, Z. Lu, S. Y. B. Boboua, J. Li, and W. Zhou (2020) Degradation of rice straw at low temperature using a novel microbial consortium LTF-27 with efficient ability. Bioresour. Technol. 304: 123064.
Zhang, D., Y. Luo, S. Chu, Y. Zhi, B. Wang, and P. Zhou (2016) Biological pretreatment of rice straw with Streptomyces griseorubens JSD-1 and its optimized production of cellulase and xylanase for improved enzymatic saccharification efficiency. Prep. Biochem. Biotechnol. 46: 575–585.
Soares, F. L., Jr., I. S. Melo, A. C. Dias, and F. D. Andreote (2012) Cellulolytic bacteria from soils in harsh environments. World J. Microbiol. Biotechnol. 28: 2195–2203.
Li, D., L. Feng, K. Liu, Y. Cheng, N. Hou, and C. Li (2016) Optimization of cold-active CMCase production by psychrotrophic Sphingomonas sp. FLX-7 from the cold region of China. Cellulose (Lond.) 23: 1335–1347.
Zhang, S., D. Shan, X. Liu, and M. Sun (2018) Cellulose-degrading strains: their screening and application to corn straw in low-temperature environments. Pol. J. Environ. Stud. 27: 2349–2355.
Rastogi, G., G. L. Muppidi, R. N. Gurram, A. Adhikari, K. M. Bischoff, S. R. Hughes, W. A. Apel, S. S. Bang, D. J. Dixon, and R. K. Sani (2009) Isolation and characterization of cellulose-degrading bacteria from the deep subsurface of the Homestake gold mine, Lead, South Dakota, USA. J. Ind. Microbiol. Biotechnol. 36: 585–598.
Legodi, L. M., D. La Grange, E. L. J. van Rensburg, and I. Ncube (2019) Isolation of cellulose degrading fungi from decaying banana pseudostem and Strelitzia alba. Enzyme Res. 2019: 1390890.
Steiner, E. and R. Margesin (2020) Production and partial characterization of a crude cold-active cellulase (CMCase) from Bacillus mycoides AR20-61 isolated from an Alpine forest site. Ann. Microbiol. 70: 67.
Gong, X., H. Zou, C. Qian, Y. Yu, Y. Hao, L. Li, Q. Wang, Y. Jiang, and J. Ma (2020) Construction of in situ degradation bacteria of corn straw and analysis of its degradation efficiency. Ann. Microbiol. 70: 62.
Peng, J., A. E.-F. Abomohra, M. Elsayed, X. Zhang, Q. Fan, and P. Ai (2019) Compositional changes of rice straw fibers after pretreatment with diluted acetic acid: towards enhanced biomethane production. J. Clean. Prod. 230: 775–782.
Montoya-Rosales, J. J., M. Peces, L. M. González-Rodríguez, F. Alatriste-Mondragón, and D. K. Villa-Gómez (2020) A broad overview comparing a fungal, thermal and acid pre-treatment of bean straw in terms of substrate and anaerobic digestion effect. Biomass Bioenergy 142: 105775.
Bhutto, A. W., K. Qureshi, K. Harijan, R. Abro, T. Abbas, A. A. Bazmi, S. Karim, and G. Yu (2017) Insight into progress in pre-treatment of lignocellulosic biomass. Energy (Oxf.) 122: 724–745.
Ursachi, V.-F. and G. Gutt (2020) Production of cellulosic ethanol from enzymatically hydrolysed wheat straws. Appl. Sci. (Basel) 10: 7638.
Sun, S., Y. Zhang, K. Liu, X. Chen, C. Jiang, M. Huang, H. Zang, and C. Li (2020) Insight into biodegradation of cellulose by psychrotrophic bacterium Pseudomonas sp. LKR-1 from the cold region of China: optimization of cold-active cellulase production and the associated degradation pathways. Cellulose (Lond.) 27: 315–333.
Teather, R. M. and P. J. Wood (1982) Use of Congo redpolysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 43: 777–780.
Kumar, S., M. Nei, J. Dudley, and K. Tamura (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9: 299–306.
Miller, G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428.
Guezzane, C. E., H. E. Moudden, H. Harhar, I. Warad, A. Bellaouchou, A. Guenbour, A. Zarrouk, and M. Tabyaoui (2021) Optimization of roasting conditions on the bioactive compounds and their antioxidant power from Opuntia fiscus-Indica seeds using response surface methodology (RSM). Biointerface Res. Appl. Chem. 11: 10510–10532.
Kumar, S., V. Kumar, and P. Gautam (2021) Response surface optimization and impact of immobilized enzymes naringianse and tannase on the quality parameters of Citrus maxima juice. Biointerface Res. Appl. Chem. 11: 11535–11552.
Ji, J., D. Yuan, C. Jin, G. Wang, X. Li, and C. Guan (2020) Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant. 42: 42.
Zhao, S. S., Y. Y. Zhang, W. Yan, L. L. Cao, Y. Xiao, and Y. H. Ye (2017) Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol. Lett. 364: https://doi.org/10.1093/femsle/fnw287.
Jiao, R., S. Munir, P. He, H. Yang, Y. Wu, J. Wang, P. He, Y. Cai, G. Wang, and Y. He (2020) Biocontrol potential of the endophytic Bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biol. Control 143: 104160.
Anguiano Cabello, J. C., A. Flores Olivas, V. Olalde Portugal, R. Arredondo Valdés, and E. I. Laredo Alcalá (2019) Evaluation of Bacillus subtilis as promoters of plant growth. Rev. Bio Cienc. 6: e418.
Jarvinen, K. T., E. S. Melin, and J. A. Puhakka (1994) High-rate bioremediation of chlorophenol-contaminated groundwater at low temperatures. Environ. Sci. Technol. 28: 2387–2392.
Zheng, G., Z. Lu, J. Li, S. Ai, and Y. Sun (2020) Screening and performance of L-14, a novel, highly efficient and low temperature-resistant cellulose-degrading strain. Int. J. Agric. Biol. Eng. 13: 247–254.
Salomão, G. S. B., J. C. Agnezi, L. B. Paulino, L. B. Hencker, T. S. de Lira, P. W. Tardioli, and L. M. Pinotti (2019) Production of cellulases by solid state fermentation using natural and pretreated sugarcane bagasse with different fungi. Biocatal. Agric. Biotechnol. 17: 1–6.
Silva-Mendoza, J., A. Gómez-Treviño, U. López-Chuken, E. A. Blanco-Gámez, L. Chávez-Guerrero, and M. E. Cantú-Cárdenas (2020) Agave leaves as a substrate for the production of cellulases by Penicillium sp. and the obtainment of reducing sugars. J. Chem. 2020: 6092165.
Zheng, Z., Q. Xu, P. Liu, F. Zhou, and J. Ouyang (2018) Enhanced inulin saccharification by self-produced inulinase from a newly isolated Penicillium sp. and its application in D-Lactic acid production. Appl. Biochem. Biotechnol. 186: 122–131.
Huang, Y.-L., Z. Li, X.-N. Liu, P.-P. Wang, and J. Zhang (2010) Screening and identification of a cold-adapted cellulase-producing bacteria. Wei Sheng Wu Xue Tong Bao 37: 637–644.
Wang, C. M., C. L. Shyu, S. P. Ho, and S. H. Chiou (2008) Characterization of a novel thermophilic, cellulose-degrading bacterium Paenibacillus sp. strain B39. Lett. Appl. Microbiol. 47: 46–53.
Prasanna, H. N., G. Ramanjaneyulu, and B. Rajasekhar Reddy (2016) Optimization of cellulase production by Penicillium sp. 3 Biotech. 6: 162.
Bai, H., H. Zi, Y. Huang, M. Han, M. Irfan, N. Liu, J. Yang, H. Wang, and X. Han (2017) Catalytic properties of carboxymethyl cellulase produced from newly isolated novel fungus Penicillium ochrochloron ZH1 in submerged fermentation. Catal. Lett. 147: 2013–2022.
Song, J., J. Gu, Y. Zhai, W. Wu, H. Wang, Z. Ruan, Y. Shi, and Y Yan (2013) Biodegradation of nicosulfuron by a Talaromyces flavus LZM1. Bioresour. Technol. 140: 243–248.
Ruan, Z., S. Zhou, S. Jiang, L. Sun, Y. Zhai, Y. Wang, C. Chen, and B. Zhao (2013) Isolation and characterization of a novel cinosulfuron degrading Kurthia sp. from a methanogenic microbial consortium. Bioresour. Technol. 147: 477–483.
Li, N., Y. Han, X. Jin, L. Wang, H. Pan, H. Rui, J. Yu, L. Yang, B. Han, and G. Qi (2019) Isolation, Identification and characteristics analysis of a straw-cellulose degradation strain adaptable for low-temperature. Yu Mi Ke Xue. 27: 159–163.
Qinggeer, J.-L. Gao, X.-F. Yu, B.-L. Zhang, Z.-G. Wang, B. Naoganchaolu, S.-P. Hu, J.-Y. Sun, M. Xie, and Z. Wang (2016) Screening of a microbial consortium with efficient corn stover degradation ability at low temperature. J. Integr. Agric. 15: 2369–2379.
Zhang, X., Qinggeer, J. Gao, X. Yu, S. Hu, B. Zhang, S. Han, and B. Feng (2021) Screening and composition of the microbial consortium with corn straw decomposition under low temperature. Nong Ye Huan Jing Ke Xue Xue Bao. 40: 1565–1574.
Sa, R., J. Gao, X. Yu, and S. Hu (2013) Screening of low temperature maize stalk decomposition microorganism. Zhongguo Nong Ye Ke Xue. 46: 4082–4090.
Liang, Y.-L., Z. Zhang, M. Wu, Y. Wu, and J.-X. Feng (2014) Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. Biomed. Res. Int. 2014: 512497.
Bai, H., H. Wang, J. Sun, M. Irfan, M. Han, Y. Huang, X. Han, and Q. Yang (2013) Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H-11 in submerged fermentation. EXCLI J. 12: 528–540.
Peñalva, M. A. and H. N. Arst Jr. (2002) Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66: 426–446.
Häkkinen, M., D. Sivasiddarthan, N. Aro, M. Saloheimo, and T. M. Pakula (2015) The effects of extracellular pH and of the transcriptional regulator PACI on the transcriptome of Trichoderma reesei. Microb. Cell Fact. 14: 63.
Espinoza-Acosta, J. L., P. I. Torres-Chávez, E. Carvajal-Millán, B. Ramirez-Wong, L. A. Bello-Pérez, and B. Montaño-Leyva (2014) Ionic liquids and organic solvents for recovering lignin from lignocellulosic biomass. Bioresources. 9: 3660–3687.
Feng, X., F. Li, Y. Xu, L. Li, F. He, Y. Feng, Q. Yuan, and D. Liu (2021) Screening of cellulase producing strains from rotten wood in Xinjiang cold area and analysis of their characteristics of enzyme production at low temperature. Zhejiang Nong Ye Xue Bao. 33: 1468–1476.
Yang, B. and C. E. Wyman (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuel. Bioprod. Biorefin. 2: 26–40.
Dziekońska-Kubczak, U., J. Berłowska, P. Dziugan, P. Patelski, K. Pielech-Przybylska, and M. Balcerek (2018) Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies (Basel) 11: 2153.
Rattanaporn, K., P. Tantayotai, T. Phusantisampan, P. Pornwongthong, and M. Sriariyanun (2018) Organic acid pretreatment of oil palm trunk: effect on enzymatic saccharification and ethanol production. Bioprocess Biosyst. Eng. 41: 467–477.
Zheng, Y., C. Lee, C. Yu, Y.-S. Cheng, R. Zhang, B. M. Jenkins, and J. S. VanderGheynst (2013) Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy 105: 1–7.
Ren, J., P. Yu, and X. Xu (2019) Straw utilization in China-status and recommendations. Sustainability. 11: 1762.
Li, Z., Z. Song, B. P. Singh, and H. Wang (2019) The impact of crop residue biochars on silicon and nutrient cycles in croplands. Sci. Total Environ. 659: 673–680.
Cavalli, E., A. Lange, C. Cavalli, and M. Behling (2018) Decomposition and release of nutrients from crop residues on soybean-maize cropping systems. Rev. Bras. Cienc. Agrar. 13: e5527.
Yan, F., Y. Sun, X. Hui, M. Jiang, K. Xiang, Y. Wu, Q. Zhang, Y. Tang, Z. Yang, Y. Sun, and M. Jun (2019) The effect of straw mulch on nitrogen, phosphorus and potassium uptake and use in hybrid rice. Paddy Water Environ. 17: 23–33.
Wu, Y., L. Wang, Y. Tang, Y. Xie, and L. Liu (2019) Effect of applying straw of Solanum photeinocarpum and their hybrids on nutrient of lettuce and soil under cadmium stress. IOP Conf. Ser. Earth Environ. Sci. 358: 022078.
Acknowledgements
This work was supported by Tianjin Science and Technology Research and Development Plan Project (Grant numbers: 19YFZCSN00280) and Tianjin Rice Industry Technology System Innovation Team Construction (Grant numbers: ITTRRS2018007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Dong, X., Ji, J., Zhang, S. et al. Study on a Low-temperature Cellulose-degrading Strain: Fermentation Optimization, Straw Degradation, and the Effect of Fermentation Broth on Seed Growth. Biotechnol Bioproc E 27, 652–667 (2022). https://doi.org/10.1007/s12257-021-0265-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-021-0265-0