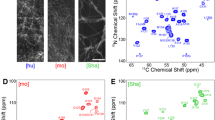
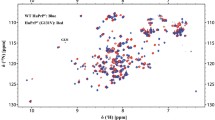
Abstract
The C-terminally truncated Y145Stop variant of prion protein (PrP23-144) has been linked to a heritable prionopathy in humans and is also capable of triggering a transmissible prion disease in mice. PrP23-144 can be converted from soluble monomeric form to amyloid under physiological conditions, providing an in vitro model for investigating the molecular basis of amyloid strains and cross-seeding barriers. Here, we use magic-angle spinning solid-state NMR to establish the sequential backbone and sidechain 13C and 15N chemical shift assignments for amyloid fibrils formed by the A117V and M129V mutants of human PrP23-144, which in the context of full length PrP in vivo are among the specific residues associated with development of Gerstmann–Straüssler–Scheinker disease. The chemical shift data are utilized to identify amino acids comprising the rigid amyloid core regions and to predict the protein secondary structures for human PrP23-144 A117V and M129V fibrils.




Similar content being viewed by others
References
Aucoin D, Xia Y, Theint T, Nadaud PS, Surewicz K, Surewicz WK, Jaroniec CP (2019) Protein-solvent interfaces in human Y145Stop prion protein amyloid fibrils probed by paramagnetic solid-state NMR spectroscopy. J Struct Biol 206(1):36–42. https://doi.org/10.1016/j.jsb.2018.04.002
Choi J-K, Cali I, Surewicz K, Kong Q, Gambetti P, Surewicz WK (2016) Amyloid fibrils from the N-terminal prion protein fragment are infectious. Proc Natl Acad Sci USA 113(48):13851–13856. https://doi.org/10.1073/pnas.1610716113
Cracco L, Xiao X, Nemani SK, Lavrich J, Cali I, Ghetti B, Notari S, Surewicz WK, Gambetti P (2019) Gerstmann-Sträussler-Scheinker disease revisited: accumulation of covalently-linked multimers of internal prion protein fragments. Acta Neuropathol Commun 7(1):85. https://doi.org/10.1186/s40478-019-0734-2
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6(3):277–293. https://doi.org/10.1007/BF00197809
Ghetti B, Piccardo P, Spillantini MG, Ichimiya Y, Porro M, Perini F, Kitamoto T, Tateishi J, Seiler C, Frangione B, Bugiani O, Giaccone G, Prelli F, Goedert M, Dlouhy SR, Tagliavini F (1996) Vascular variant of prion protein cerebral amyloidosis with tau-positive neurofibrillary tangles: the phenotype of the stop codon 145 mutation in PRNP. Proc Natl Acad Sci 93(2):744–748. https://doi.org/10.1073/pnas.93.2.744
Goddard TD, Kneller DG (2006) SPARKY 3. University of California, San Francisco
Helmus JJ, Surewicz K, Nadaud PS, Surewicz WK, Jaroniec CP (2008) Molecular conformation and dynamics of the Y145Stop variant of human prion protein in amyloid fibrils. Proc Natl Acad Sci 105(17):6284–6289. https://doi.org/10.1073/pnas.0711716105
Helmus JJ, Surewicz K, Surewicz WK, Jaroniec CP (2010) Conformational flexibility of the Y145Stop human prion protein amyloid fibrils probed by solid-state nuclear magnetic resonance spectroscopy. J Am Chem Soc 132(7):2393–2403. https://doi.org/10.1021/ja909827v
Helmus JJ, Surewicz K, Apostol MI, Surewicz WK, Jaroniec CP (2011) Intermolecular alignment in Y145Stop human prion protein amyloid fibrils probed by solid-state NMR spectroscopy. J Am Chem Soc 133(35):13934–13937. https://doi.org/10.1021/ja206469q
Jones EM, Surewicz K, Surewicz WK (2006) Role of N-terminal familial mutations in prion protein fibrillization and prion amyloid propagation in vitro. J Biol Chem 281(12):8190–8196. https://doi.org/10.1074/jbc.M513417200
Jones EM, Wu B, Surewicz K, Nadaud PS, Helmus JJ, Chen S, Jaroniec CP, Surewicz WK (2011) Structural polymorphism in amyloids: new insights from studies with Y145Stop prion protein fibrils. J Biol Chem 286:42777–42784. https://doi.org/10.1074/jbc.M111.302539
Kitamoto T, Iizuka R, Tateishi J (1993) An amber mutation of prion protein in Gerstmann-Sträussler syndrome with mutant PrP plaques. Biochem Biophys Res Commun 192(2):525–531. https://doi.org/10.1006/bbrc.1993.1447
Kundu B, Maiti NR, Jones EM, Surewicz KA, Vanik DL, Surewicz WK (2003) Nucleation-dependent conformational conversion of the Y145Stop variant of human prion protein: structural clues for prion propagation. Proc Natl Acad Sci 100(21):12069–12074. https://doi.org/10.1073/pnas.2033281100
Piccardo P, Liepnieks JJ, William A, Dlouhy SR, Farlow MR, Young K, Nochlin D, Bird TD, Nixon RR, Ball MJ, DeCarli C, Bugiani O, Tagliavini F, Benson MD, Ghetti B (2001) Prion proteins with different conformations accumulate in Gerstmann-Sträussler-Scheinker disease caused by A117V and F198S mutations. Am J Pathol 158(6):2201–2207. https://doi.org/10.1016/S0002-9440(10)64692-5
Pirisinu L, Di Bari MA, D’Agostino C, Marcon S, Riccardi G, Poleggi A, Cohen ML, Appleby BS, Gambetti P, Ghetti B, Agrimi U, Nonno R (2016) Gerstmann-Sträussler-Scheinker disease subtypes efficiently transmit in bank voles as genuine prion diseases. Sci Rep 6:20443. https://doi.org/10.1038/srep20443
Prusiner SB (1998) Prions. Proc Natl Acad Sci 95(23):13363–13383. https://doi.org/10.1073/pnas.95.23.13363
Shannon MD, Theint T, Mukhopadhyay D, Surewicz K, Surewicz WK, Marion D, Schanda P, Jaroniec CP (2019) Conformational dynamics in the core of human Y145Stop prion protein amyloid probed by relaxation dispersion NMR. ChemPhysChem 20(2):311–317. https://doi.org/10.1002/cphc.201800779
Shen Y, Bax A (2013) Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 56(3):227–241. https://doi.org/10.1007/s10858-013-9741-y
Surewicz WK, Jones EM, Apetri AC (2006) The emerging principles of mammalian prion propagation and transmissibility barriers: insight from studies in vitro. Acc Chem Res 39(9):654–662. https://doi.org/10.1021/ar050226c
Tagliavini F, Lievens PM-J, Tranchant C, Warter J-M, Mohr M, Giaccone G, Perini F, Rossi G, Salmona M, Piccardo P, Ghetti B, Beavis RC, Bugiani O, Frangione B, Prelli F (2001) A 7-kDa Prion Protein (PrP) fragment, an integral component of the PrP region required for infectivity, is the major amyloid protein in Gerstmann-Sträussler-Scheinker disease A117V. J Biol Chem 276(8):6009–6015. https://doi.org/10.1074/jbc.M007062200
Theint T, Nadaud PS, Aucoin D, Helmus JJ, Pondaven SP, Surewicz K, Surewicz WK, Jaroniec CP (2017) Species-dependent structural polymorphism of Y145Stop prion protein amyloid revealed by solid-state NMR spectroscopy. Nat Commun 8(1):753. https://doi.org/10.1038/s41467-017-00794-z
Theint T, Nadaud PS, Surewicz K, Surewicz WK, Jaroniec CP (2017) 13C and 15N chemical shift assignments of mammalian Y145Stop prion protein amyloid fibrils. Biomol NMR Assign 11(1):75–80. https://doi.org/10.1007/s12104-016-9723-6
Theint T, Xia Y, Nadaud PS, Mukhopadhyay D, Schwieters CD, Surewicz K, Surewicz WK, Jaroniec CP (2018) Structural studies of amyloid fibrils by paramagnetic solid-state nuclear magnetic resonance spectroscopy. J Am Chem Soc 140(41):13161–13166. https://doi.org/10.1021/jacs.8b06758
Vanik DL, Surewicz KA, Surewicz WK (2004) Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol Cell 14(1):139–145. https://doi.org/10.1016/s1097-2765(04)00155-8
Young K, Clark HB, Piccardo P, Dlouhy SR, Ghetti B (1997) Gerstmann–Sträussler–Scheinker disease with the PRNP P102L mutation and valine at codon 129. Mol Brain Res 44(1):147–150. https://doi.org/10.1016/S0169-328X(96)00251-3
Acknowledgements
This research was supported by NIH (Grants R01GM094357 and S10OD012303 to C.P.J., P01AI106705 and R01NS083687 to W.K.S., and RF1AG061797 to W.K.S. and C.P.J.), and NSF (Grant MCB-1715174 to C.P.J.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accession numbers
Chemical shift assignments for A117V and M129V huPrP23-144 amyloid fibrils have been deposited in the BioMagResBank (https://www.bmrb.wisc.edu) under accession numbers 50,312 and 50,313, respectively.
Rights and permissions
About this article
Cite this article
Dao, H.H., Hlaing, M.Z., Ma, Y. et al. 13C and 15N chemical shift assignments of A117V and M129V human Y145Stop prion protein amyloid fibrils. Biomol NMR Assign 15, 45–51 (2021). https://doi.org/10.1007/s12104-020-09981-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-020-09981-4