Abstract
Background
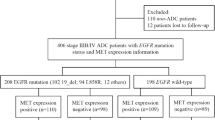
About 50–60% treatment-naïve advanced non-small-cell lung cancers were coexistence of epidermal growth factor receptor (EGFR) and mesenchymal epithelial transition (MET) overexpression. However, few studies demonstrated the prognostic value of MET protein expression in untreated EGFR-mutant lung adenocarcinoma (LUAD).
Methods
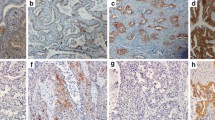
A total of 235 EGFR-mutant untreated advanced LUAD patients were retrospectively enrolled. MET expression was determined using immunohistochemistry, and MET positivity was defined as 2 + or 3 + using the METmab scoring algorithm. Progression-free survival (PFS) and overall survival (OS) were analysed according to MET expression status. Independent factors predicting prognosis were identified using multivariate Cox regression analyses.
Results
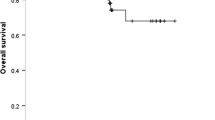
Of the 235 patients, 113 (48.1%) harboured exon 19 deletion (19_del), 103 (43.8%) had exon 21 L858R mutations, and 19 (8.1%) had other mutation types, including exon 21 L861Q, exon 18 G719A/C, exon 20 S768I, and L858R/19_del double mutations. MET-positive expression was observed in 192 (81.7%) cases. There was no significant difference in baseline clinicopathological characteristics between MET positivity and MET negativity groups. Patients were stratified by different EGFR mutation subtypes. MET-positive patients in the L858R mutation subgroup had markedly shorter PFS and OS than MET-negative patients (median PFS: 13 versus 27.5 months, p < 0.001; median OS: 29 versus not reached, p = 0.008), but no significant difference was observed in the 19_del subgroup. Multivariate Cox regression analyses indicated that MET positivity was an independent predictor for poor PFS and OS in L858R subgroup (PFS: HR = 3.059, 95% CI 1.552–6.029, p = 0.001; OS: HR = 3.511, 95% CI 1.346–9.160, p = 0.010). Additionally, an inferior survival outcome of MET positivity was observed in the L858R mutation subgroup when treated with EGFR–tyrosine kinase inhibitor (TKI) monotherapy as the first-line regimen (median PFS: 13 versus 36.5 months, p < 0.001; median OS: 29 versus not reached, p = 0.012) but not with EGFR–TKI plus platinum doublet chemotherapy.
Conclusions
MET positive expression was an independent predictor of poor outcomes in untreated EGFR L858R mutation advanced LUAD patients treated with first-line EGFR–TKI monotherapy.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(5):497–530.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022. https://doi.org/10.3322/caac.21731.
Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. 2018;17(1):53. https://doi.org/10.1186/s12943-018-0793-1.
Nagano T, Tachihara M, Nishimura Y. Mechanism of Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors and a Potential Treatment Strategy. Cells. 2018. https://doi.org/10.3390/cells7110212.
Passaro A, Janne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2(4):377–91. https://doi.org/10.1038/s43018-021-00195-8.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. https://doi.org/10.1126/science.1141478.
Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105(2):692–7. https://doi.org/10.1073/pnas.0707270105.
Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(5):739–42. https://doi.org/10.1001/jamaoncol.2018.0049.
Liang H, Li C, Zhao Y, Zhao S, Huang J, Cai X, et al. Concomitant Mutations in EGFR 19Del/L858R Mutation and Their Association with Response to EGFR-TKIs in NSCLC Patients. Cancer Manag Res. 2020;12:8653–62. https://doi.org/10.2147/CMAR.S255967.
Guo R, Berry LD, Aisner DL, Sheren J, Boyle T, Bunn PA Jr, et al. MET IHC Is a Poor Screen for MET Amplification or MET Exon 14 Mutations in Lung Adenocarcinomas: Data from a Tri-Institutional Cohort of the Lung Cancer Mutation Consortium. J Thorac Oncol. 2019;14(9):1666–71. https://doi.org/10.1016/j.jtho.2019.06.009.
Li A, Niu FY, Han JF, Lou NN, Yang JJ, Zhang XC, et al. Predictive and prognostic value of de novo MET expression in patients with advanced non-small-cell lung cancer. Lung Cancer. 2015;90(3):375–80. https://doi.org/10.1016/j.lungcan.2015.10.021.
Tsakonas G, Botling J, Micke P, Rivard C, LaFleur L, Mattsson J, et al. c-MET as a biomarker in patients with surgically resected non-small cell lung cancer. Lung Cancer. 2019;133:69–74. https://doi.org/10.1016/j.lungcan.2019.04.028.
Huang L, An SJ, Chen ZH, Su J, Yan HH, Wu YL. MET expression plays differing roles in non-small-cell lung cancer patients with or without EGFR mutation. J Thorac Oncol. 2014;9(5):725–8. https://doi.org/10.1097/JTO.0000000000000105.
Raghav K, Bailey AM, Loree JM, Kopetz S, Holla V, Yap TA, et al. Untying the gordion knot of targeting MET in cancer. Cancer Treat Rev. 2018;66:95–103. https://doi.org/10.1016/j.ctrv.2018.04.008.
Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J Thorac Oncol. 2017;12(1):15–26. https://doi.org/10.1016/j.jtho.2016.10.014.
Scagliotti G, Moro-Sibilot D, Kollmeier J, Favaretto A, Cho EK, Grosch H, et al. A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for EGFR Mutation-Positive NSCLC Patients. J Thorac Oncol. 2020;15(1):80–90. https://doi.org/10.1016/j.jtho.2019.10.003.
Koeppen H, Yu W, Zha J, Pandita A, Penuel E, Rangell L, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib+/-onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res. 2014;20(17):4488–98. https://doi.org/10.1158/1078-0432.CCR-13-1836.
Kang SJ, Cho YR, Park GM, Ahn JM, Han SB, Lee JY, et al. Predictors for functionally significant in-stent restenosis: an integrated analysis using coronary angiography, IVUS, and myocardial perfusion imaging. JACC Cardiovasc Imaging. 2013;6(11):1183–90. https://doi.org/10.1016/j.jcmg.2013.09.006.
Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Mocci S, Phan S, et al. Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J Clin Oncol. 2017;35(4):412–20. https://doi.org/10.1200/JCO.2016.69.2160.
Wu YL, Zhang L, Kim DW, Liu X, Lee DH, Yang JC, et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(31):3101–9. https://doi.org/10.1200/JCO.2018.77.7326.
Bauml J, Cho BC, Park K, Lee KH, Cho EK, Kim DW, et al. Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naive EGFR mutant (EGFRm) non-small cell lung cancer (NSCLC) and potential biomarkers for response. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.2021.39.15_suppl.9006.
Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507–16. https://doi.org/10.1016/j.annonc.2020.01.013.
Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. 2020;8(11):1132–43. https://doi.org/10.1016/S2213-2600(20)30154-5.
Wang N, Zhu Y, Wu Y, Huang B, Wu J, Zhang R, et al. MET overexpression in EGFR L858R mutant treatment-naive advanced lung adenocarcinoma correlated with poor prognosis: a real-world retrospective study. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04225-5.
Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26(10):1742–51. https://doi.org/10.1200/JCO.2007.12.1178.
Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med. 2020;383(10):931–43. https://doi.org/10.1056/NEJMoa2004407.
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(10):944–57. https://doi.org/10.1056/NEJMoa2002787.
Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr, Blumenschein GR Jr, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(32):4105–14. https://doi.org/10.1200/JCO.2012.47.4189.
Hartmaier R, Han JY, Cho BC, Markovets A, Kurian N, Cantarini M, et al. Tumor response and MET-detection methods exploratory biomarker analysis of Part B of the Ph 1b TATTON study. Cancer Res. 2021;81(13):2.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China [Grant number 82072333]; and the Natural Science Foundation of Hubei Province [Grant number 2023AFB986]; and the National Key Research and Development Program of China [Grant number 2022YFF1203300].
Author information
Authors and Affiliations
Contributions
NW: methodology, writing—original draft, formal analysis. YZ: methodology, conceptualization. JW: formal analysis, resources. YZ: investigation, data curation. YW: investigation. BH: resources, project administration. RZ: resources. JF: conceptualization, supervision, project administration. XN: conceptualization, supervision, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethics approval and consent to participate
Approval was obtained from the ethics committee of Tongji Medical College of Huazhong University of Science and Technology, and all patients signed informed consent forms. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Zhang, Y., Wu, J. et al. MET overexpression correlated with prognosis of EGFR-mutant treatment‑naïve advanced lung adenocarcinoma: a real‑world retrospective study. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03391-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03391-x