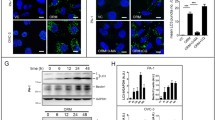
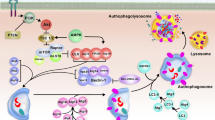
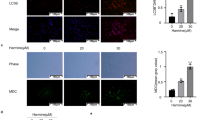
Abstract
Ovarian cancer (OC) is the most deadly tumor that may develop in a woman's reproductive system. It is also one of the most common causes of death among those who have been diagnosed with cancer in women. An adapter protein known as sequestosome 1(SQSTM1) or p62 is primarily responsible for the transportation, degradation, and destruction of a wide variety of proteins. This adapter protein works in conjunction with the autophagy process as well as the ubiquitin proteasome degradation pathway. In addition, the ability of SQSTM1 to interact with multiple binding partners link SQSTM1 to various pathways in the context of antioxidant defense system and inflammation. In this review, we outline the processes underlying the control that SQSTM1 has on these pathways and how their dysregulation contributes to the development of OC. At the final, the therapeutic approaches based on SQSTM1 targeting have been discussed.

Similar content being viewed by others
Data availability
It is not applicable.
References
Nasioudis D, Sisti G, Kanninen TT, Holcomb K, Di Tommaso M, Fambrini M, et al. Epidemiology and outcomes of squamous ovarian carcinoma; a population-based study. Gynecol Oncol. 2016;141(1):128–33.
Wang W, Li T, Gao J, editors. Diagnosis of cervical cancer by ultrasound graduates using intelligent three-dimensional imaging. Eur J Gynaecol Oncol. 2022: MRE Press 14 Robinson Rd# 08-01A FAR East Finance, Singapore, Singapore.
Yang D, editor Clinical effect of hysteroscopic surgery in the treatment of endometrial polyps. Indian Journal of Pharmaceutical Sciences; 2021: Indian Pharmaceutical Assoc Kalina, Santa Cruz East, Mumbai, 00000, India.
Bai H, Li H, Li W, Gui T, Yang J, Cao D, et al. The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines. Oncotarget. 2015;6(28):25520.
Orfanelli T, Jeong J, Doulaveris G, Holcomb K, Witkin S. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int J Cancer. 2014;135(3):519–28.
Sene AA, Zandieh Z, Soflaei M, Torshizi HM, Sheibani K. Using artificial intelligence to predict the intrauterine insemination success rate among infertile couples. Middle East Fertility Soc J. 2021;26(1):1–7.
Sharbatoghli M, Vafaei S, Aboulkheyr Es H, Asadi-Lari M, Totonchi M, Madjd Z. Prediction of the treatment response in ovarian cancer: a ctDNA approach. J Ovarian Res. 2020;13:1–12.
Kalid O, Gotliv I, Levy-Apter E, Beker DF, Cherniavsky-Lev M, Rotem E, et al. PTX80, a novel compound targeting the autophagy receptor p62/SQSTM1 for treatment of cancer. Chem Biol Drug Des. 2022;100(5):623–38.
Huang X, Chen J, Xiang H, Yu X. Maslinic acid suppresses cervical cancer growth by inducing apoptosis through a P53-dependent and Bcl related pathway in vitro. J Biol Regul Homeostatic Agents. 2022;36(3):557–63.
Vafaei S, Fattahi F, Sahlolbei M, Kiani J, Yazdanpanah A, Madjd Z. Dynamic signature of tRNA-derived small RNAs in cancer pathogenesis as a promising valuable approach. Crit Rev™ Eukaryotic Gene Expr. 2020;30(5):1.
Alegre F, Moragrega ÁB, Polo M, Marti-Rodrigo A, Esplugues JV, Blas-Garcia A, et al. Role of p62/SQSTM1 beyond autophagy: a lesson learned from drug-induced toxicity in vitro. Br J Pharmacol. 2018;175(3):440–55.
Ciuffa R, Lamark T, Tarafder AK, Guesdon A, Rybina S, Hagen WJ, et al. The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep. 2015;11(5):748–58.
Ishimura R, Tanaka K, Komatsu M. Dissection of the role of p62/Sqstm1 in activation of Nrf2 during xenophagy. FEBS Lett. 2014;588(5):822–8.
Sui XZ. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371.
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen H-Y, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–75.
Tang J, Li Y, Xia S, Li J, Yang Q, Ding K, et al. Sequestosome 1/p62: a multitasker in the regulation of malignant tumor aggression (review). Int J Oncol. 2021;59(4):1.
Yazdani Z, Rafiei A, Golpour M, Zafari P, Moonesi M, Ghaffari S. IL-35, a double-edged sword in cancer. J Cell Biochem. 2020;121(3):2064–76.
Sánchez-Martín P, Komatsu M. p62/SQSTM1-steering the cell through health and disease. J Cell Sci. 2018;131(21):jcs222836.
Moscat J, Karin M, Diaz-Meco MT. p62 in cancer: signaling adaptor beyond autophagy. Cell. 2016;167(3):606–9.
Deretic V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol. 2012;24(1):21–31.
Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, et al. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev Cell. 2016;37(4):337–49.
Pölönen P, Jawahar Deen A, Leinonen HM, Jyrkkänen H-K, Kuosmanen S, Mononen M, et al. Nrf2 and SQSTM1/p62 jointly contribute to mesenchymal transition and invasion in glioblastoma. Oncogene. 2019;38(50):7473–90.
Nguyen TD, Shaid S, Vakhrusheva O, Koschade SE, Klann K, Thölken M, et al. Loss of the selective autophagy receptor p62 impairs murine myeloid leukemia progression and mitophagy. Blood J Am Soc Hematol. 2019;133(2):168–79.
Karras P, Riveiro-Falkenbach E, Cañón E, Tejedo C, Calvo TG, Martínez-Herranz R, et al. p62/SQSTM1 fuels melanoma progression by opposing mRNA decay of a selective set of pro-metastatic factors. Cancer Cell. 2019;35(1):46–63. e10.
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–84.
Lam HC, Baglini CV, Lope AL, Parkhitko AA, Liu H-J, Alesi N, et al. p62/SQSTM1 cooperates with hyperactive mTORC1 to regulate glutathione production, maintain mitochondrial integrity, and promote tumorigenesis. Can Res. 2017;77(12):3255–67.
Umemura A, He F, Taniguchi K, Nakagawa H, Yamachika S, Font-Burgada J, et al. p62, upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell. 2016;29(6):935–48.
Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26(1):121–35.
Huang J, Duran A, Reina-Campos M, Valencia T, Castilla EA, Müller TD, et al. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33(4):770–84. e6.
Overå KS, Garcia-Garcia J, Bhujabal Z, Jain A, Øvervatn A, Larsen KB, et al. TRIM32, but not its muscular dystrophy-associated mutant, positively regulates and is targeted to autophagic degradation by p62/SQSTM1. J Cell Sci. 2019;132(23):jcs236596.
Sparrer KM, Gableske S, Zurenski MA, Parker ZM, Full F, Baumgart GJ, et al. TRIM23 mediates virus-induced autophagy via activation of TBK1. Nat Microbiol. 2017;2(11):1543–57.
Zaffagnini G, Savova A, Danieli A, Romanov J, Tremel S, Ebner M, et al. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 2018;37(5):e98308.
Kitamura H, Motohashi H. NRF2 addiction in cancer cells. Cancer Sci. 2018;109(4):900–11.
Ichimura Y, Waguri S, Sou Y-s, Kageyama S, Hasegawa J, Ishimura R, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51(5):618–31.
Ma S, Attarwala IY, Xie X-Q. SQSTM1/p62: a potential target for neurodegenerative disease. ACS Chem Neurosci. 2019;10(5):2094–114.
Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15(11):1139–53.
Zi D, Zhou Z-W, Yang Y-J, Huang L, Zhou Z-L, He S-M, et al. Danusertib induces apoptosis, cell cycle arrest, and autophagy but inhibits epithelial to mesenchymal transition involving PI3K/Akt/mTOR signaling pathway in human ovarian cancer cells. Int J Mol Sci. 2015;16(11):27228–51.
Bahrami F, Pourgholami MH, Mekkawy AH, Rufener L, Morris DL. Monepantel induces autophagy in human ovarian cancer cells through disruption of the mTOR/p70S6K signalling pathway. Am J Cancer Res. 2014;4(5):558.
Xu L, Zhang X, Li Y, Lu S, Lu S, Li J, et al. Neferine induces autophagy of human ovarian cancer cells via p38 MAPK/JNK activation. Tumor Biol. 2016;37:8721–9.
Yang X, Xiang X, Xia M, Su J, Wu Y, Shen L, et al. Inhibition of JNK3 promotes apoptosis induced by BH3 mimetic S1 in chemoresistant human ovarian cancer cells. Anat Rec. 2015;298(2):386–95.
Guo JY, Chen H-Y, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–70.
Li S, Wei Y. Association of HMGB1, BRCA1 and P62 expression in ovarian cancer and chemotherapy sensitivity. Oncol Lett. 2018;15(6):9572–6.
Ponomarenko DM, Klimova ID, Chapygina YA, Dvornichenko VV, Zhukova NV, Orlova RV, et al. Safety and efficacy of p62 DNA vaccine ELENAGEN in a first-in-human trial in patients with advanced solid tumors. Oncotarget. 2017;8(32):53730.
Wang J, Garbutt C, Ma H, Gao P, Hornicek FJ, Kan Q, et al. Expression and role of autophagy-associated p62 (SQSTM1) in multidrug resistant ovarian cancer. Gynecol Oncol. 2018;150(1):143–50.
Bartsch G, Jennewein L, Harter PN, Antonietti P, Blaheta RA, Kvasnicka H-M, et al. Autophagy-associated proteins BAG3 and p62 in testicular cancer. Oncol Rep. 2016;35(3):1629–35.
Iwadate R, Inoue J, Tsuda H, Takano M, Furuya K, Hirasawa A, et al. High expression of p62 protein is associated with poor prognosis and aggressive phenotypes in endometrial cancer. Am J Pathol. 2015;185(9):2523–33.
Darvekar SR, Elvenes J, Brenne HB, Johansen T, Sjøttem E. SPBP is a sulforaphane induced transcriptional coactivator of NRF2 regulating expression of the autophagy receptor p62/SQSTM1. PLoS ONE. 2014;9(1): e85262.
Iwadate R, Inoue J, Tsuda H, Takano M, Furuya K, Hirasawa A, et al. High expression of SQSTM1/p62 protein is associated with poor prognosis in epithelial ovarian cancer. Acta Histochem Cytochem. 2014:14048.
Xia M-h, Yan X-y, Zhou L, Xu L, Zhang L-c, Yi H-w, et al. p62 suppressed VK3-induced oxidative damage through Keap1/Nrf2 pathway in human ovarian cancer cells. J Cancer. 2020;11(6):1299.
Yan XY, Zhong XR, Yu SH, Zhang LC, Liu YN, Zhang Y, et al. p62 aggregates mediated Caspase 8 activation is responsible for progression of ovarian cancer. J Cell Mol Med. 2019;23(6):4030–42.
Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, et al. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell. 2011;44(1):134–46.
Sanz L, Diaz-Meco MT, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-κB activation by the IL-1-TRAF6 pathway. EMBO J. 2000;19(7):1576–86.
Zheng J, Long X, Chen H, Ji Z, Shu B, Yue R, et al. Photoclick reaction constructs glutathione-responsive theranostic system for anti-tuberculosis. Front Mol Biosci. 2022;9:39.
Omidi N, Arabloo J, Rezapour A, Alaeddini F, Bragazzi NL, Pourasghari H, et al. Burden of hypertensive heart disease in Iran during 1990–2017: findings from the Global Burden of Disease study 2017. PLoS ONE. 2021;16(9): e0257617.
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–23.
Rogov V, Dötsch V, Johansen T, Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014;53(2):167–78.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24.
de la Vega MR, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34(1):21–43.
Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25(5):1037–43.
Zhang X-Y, Zhang M, Cong Q, Zhang M-X, Zhang M-Y, Lu Y-Y, et al. Hexokinase 2 confers resistance to cisplatin in ovarian cancer cells by enhancing cisplatin-induced autophagy. Int J Biochem Cell Biol. 2018;95:9–16.
Lou J-S, Zhao L-P, Huang Z-H, Chen X-Y, Xu J-T, Tai WC-S, et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021;80:153370.
Periyasamy-Thandavan S, Jiang M, Schoenlein P, Dong Z. Autophagy: molecular machinery, regulation, and implications for renal pathophysiology. Am J Physiol Renal Physiol. 2009;297(2):F244–56.
Ma L, Xu Y, Su J, Yu H, Kang J, Li H, et al. Autophagic flux promotes cisplatin resistance in human ovarian carcinoma cells through ATP-mediated lysosomal function. Int J Oncol. 2015;47(5):1890–900.
Yan XY, Zhang Y, Zhang JJ, Zhang LC, Liu YN, Wu Y, et al. p62/SQSTM 1 as an oncotarget mediates cisplatin resistance through activating RIP 1-NF-κB pathway in human ovarian cancer cells. Cancer Sci. 2017;108(7):1405–13.
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–31.
Sekine S, Youle RJ. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 2018;16(1):1–12.
Wei H, Wang C, Croce CM, Guan J-L. p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 2014;28(11):1204–16.
Zhang X, Qu Y-Y, Liu L, Qiao Y-N, Geng H-R, Lin Y, et al. Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylation. Cell Rep. 2021;37(2):109821.
Cai PC, Shi L, Liu VW, Tang HW, Liu IJ, Leung TH, et al. Elevated TAK1 augments tumor growth and metastatic capacities of ovarian cancer cells through activation of NF-κB signaling. Oncotarget. 2014;5(17):7549.
Samimi Z, Kardideh B, Zafari P, Bahrehmand F, Roghani SA, Taghadosi M. The impaired gene expression of adenosine monophosphate-activated kinase (AMPK), a key metabolic enzyme in leukocytes of newly diagnosed rheumatoid arthritis patients. Mol Biol Rep. 2019;46:6353–60.
Marty P, Chatelain B, Lihoreau T, Tissot M, Dirand Z, Humbert P, et al. Halofuginone regulates keloid fibroblast fibrotic response to TGF-β induction. Biomed Pharmacother. 2021;135: 111182.
Stępkowski TM, Kruszewski MK. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radical Biol Med. 2011;50(9):1186–95.
Bao L, Wu J, Dodson M, Rojo de la Vega EM, Ning Y, Zhang Z, et al. ABCF2, an Nrf2 target gene, contributes to cisplatin resistance in ovarian cancer cells. Mol Carcinogenesis. 2017;56(6):1543–53.
Wu J, Bao L, Zhang Z, Yi X. Nrf2 induces cisplatin resistance via suppressing the iron export related gene SLC40A1 in ovarian cancer cells. Oncotarget. 2017;8(55):93502.
Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285(29):22576–91.
Xia M, Yu H, Gu S, Xu Y, Su J, Li H, et al. p62/SQSTM1 is involved in cisplatin resistance in human ovarian cancer cells via the Keap1-Nrf2-ARE system. Int J Oncol. 2014;45(6):2341–8.
O’Mealey GB, Plafker KS, Berry WL, Janknecht R, Chan JY, Plafker SM. A PGAM5–KEAP1–Nrf2 complex is required for stress-induced mitochondrial retrograde trafficking. J Cell Sci. 2017;130(20):3467–80.
Kim H-RC, Najy AJ, Kim S, Kwon YT. SQSTM1/p62 as a therapeutic target in cancer. Autophagy Rep. 2022;1(1):70–4.
Hu S, Hui Z, Lirussi F, Garrido C, Ye X-Y, Xie T. Small molecule DNA-PK inhibitors as potential cancer therapy: a patent review (2010–present). Expert Opin Ther Pat. 2021;31(5):435–52.
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21(1):144.
Yeh L-Y, Liu C-J, Wong Y-K, Chang C, Lin S-C, Chang K-W. miR-372 inhibits p62 in head and neck squamous cell carcinoma in vitro and in vivo. Oncotarget. 2015;6(8):6062.
Lee M, Nam HY, Kang H-B, Lee WH, Lee G-H, Sung G-J, et al. Epigenetic regulation of p62/SQSTM1 overcomes the radioresistance of head and neck cancer cells via autophagy-dependent senescence induction. Cell Death Dis. 2021;12(3):250.
Deng D, Luo K, Liu H, Nie X, Xue L, Wang R, et al. p62 acts as an oncogene and is targeted by miR-124-3p in glioma. Cancer Cell Int. 2019;19(1):280.
Qi J-L, He J-R, Liu C-B, Jin S-M, Yang X, Bai H-M, et al. SQSTM1/p62 regulate breast cancer progression and metastasis by inducing cell cycle arrest and regulating immune cell infiltration. Genes Dis. 2022;9(5):1332–44.
Duan C, Deng H, Xiao S, Xie J, Li H, Zhao X, et al. Accelerate gas diffusion-weighted MRI for lung morphometry with deep learning. Eur Radiol. 2022;32:702–13.
Hiruma Y, Honjo T, Jelinek DF, Windle JJ, Shin J, Roodman GD, et al. Increased signaling through p62 in the marrow microenvironment increases myeloma cell growth and osteoclast formation. Blood J Am Soc Hematol. 2009;113(20):4894–902.
Wang JL, Wang JJ, Cai ZN, Xu CJ. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62. Int J Mol Med. 2018;42(5):2481–8.
Xu L, Li S, Zhou W, Kang Z, Zhang Q, Kamran M, et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene. 2017;36(3):304–17.
Yeo SK, Wen J, Chen S, Guan J-L. Autophagy differentially regulates distinct breast cancer stem-like cells in murine models via EGFR/Stat3 and Tgfβ/Smad signaling regulation of distinct breast cancer stem cells by autophagy. Can Res. 2016;76(11):3397–410.
Venanzi F, Shifrin V, Sherman MY, Gabai V, Kiselev O, Komissarov A, et al. Broad-spectrum anti-tumor and anti-metastatic DNA vaccine based on p62-encoding vector. Oncotarget. 2013;4(10):1829.
Mohamed A, Ayman A, Deniece J, Wang T, Kovach C, Siddiqui MT, et al. P62/Ubiquitin IHC expression correlated with clinicopathologic parameters and outcome in gastrointestinal carcinomas. Front Oncol. 2015;5:70.
Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103(4):760–6.
Schläfli AM, Adams O, Galván JA, Gugger M, Savic S, Bubendorf L, et al. Prognostic value of the autophagy markers LC3 and p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget. 2016;7(26):39544–55.
Thompson HG, Harris JW, Wold BJ, Lin F, Brody JP. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22(15):2322–33.
Luo RZ, Yuan ZY, Li M, Xi SY, Fu J, He J. Accumulation of p62 is associated with poor prognosis in patients with triple-negative breast cancer. Onco Targets Ther. 2013;6:883–8.
Ju LL, Zhao CY, Ye KF, Yang H, Zhang J. Expression and clinical implication of Beclin1, HMGB1, p62, survivin, BRCA1 and ERCC1 in epithelial ovarian tumor tissues. Eur Rev Med Pharmacol Sci. 2016;20(10):1993–2003.
Inui T, Chano T, Takikita-Suzuki M, Nishikawa M, Yamamoto G, Okabe H. Association of p62/SQSTM1 excess and oral carcinogenesis. PLoS ONE. 2013;8(9):e74398.
Kuo WL, Sharifi MN, Lingen MW, Ahmed O, Liu J, Nagilla M, et al. p62/SQSTM1 accumulation in squamous cell carcinoma of head and neck predicts sensitivity to phosphatidylinositol 3-kinase pathway inhibitors. PLoS ONE. 2014;9(3):e90171.
Saito T, Ichimura Y, Taguchi K, Suzuki T, Mizushima T, Takagi K, et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat Commun. 2016;7:12030.
Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51(5):618–31.
Yan X-Y, Qu X-Z, Xu L, Yu S-H, Tian R, Zhong X-R, et al. Insight into the role of p62 in the cisplatin resistant mechanisms of ovarian cancer. Cancer Cell Int. 2020;20(1):128.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
MR and MN contributed to the idea design and literature search. MN and SMG wrote parts of the manuscript. SNG contributed to designing the figure.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
It is not applicable.
Informed consent
It is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nurzadeh, M., Ghalandarpoor-Attar, S.M., Ghalandarpoor-Attar, S.N. et al. The sequestosome 1 protein: therapeutic vulnerabilities in ovarian cancer. Clin Transl Oncol 25, 2783–2792 (2023). https://doi.org/10.1007/s12094-023-03148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03148-y