Abstract
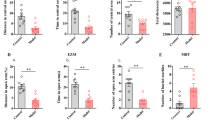
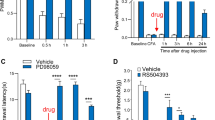
Previous studies have shown that the C-C motif chemokine ligand 2 (CCL2) is widely expressed in the nervous system and involved in regulating the development of chronic pain and related anxiety-like behaviors, but its precise mechanism is still unclear. This paper provides an in-depth examination of the involvement of CCL2-CCR2 signaling in the anterior cingulate cortex (ACC) in intraplantar injection of complete Freund’s adjuvant (CFA) leading to inflammatory pain and its concomitant anxiety-like behaviors by modulation of glutamatergic N-methyl-D-aspartate receptor (NMDAR). Our findings suggest that local bilateral injection of CCR2 antagonist in the ACC inhibits CFA-induced inflammatory pain and anxiety-like behavior. Meanwhile, the expression of CCR2 and CCL2 was significantly increased in ACC after 14 days of intraplantar injection of CFA, and CCR2 was mainly expressed in excitatory neurons. Whole-cell patch-clamp recordings showed that the CCR2 inhibitor RS504393 reduced the frequency of miniature excitatory postsynaptic currents (mEPSC) in ACC, and CCL2 was involved in the regulation of NMDAR-induced current in ACC neurons in the pathological state. In addition, local injection of the NR2B inhibitor of NMDAR subunits, Ro 25-6981, attenuated the effects of CCL2-induced hyperalgesia and anxiety-like behavior in the ACC. In summary, CCL2 acts on CCR2 in ACC excitatory neurons and participates in the regulation of CFA-induced pain and related anxiety-like behaviors through upregulation of NR2B. CCR2 in the ACC neuron may be a potential target for the treatment of chronic inflammatory pain and pain-related anxiety.






Similar content being viewed by others
Data Availability
The datasets used and/or analyzed in this study are available from the corresponding authors on reasonable request.
Abbreviations
- CCL2:
-
The C–C motif chemokine ligand 2
- CCR2:
-
The C–C motif chemokine receptor 2
- ACC:
-
Anterior cingulate cortex
- mEPSCs:
-
Miniature excitatory postsynaptic currents
- CFA:
-
Complete Freund’s adjuvant
- NMDAR:
-
NMDA receptor
References
Aydede M (2019) Does the IASP definition of pain need updating? Pain Rep 4(5):e777. https://doi.org/10.1097/PR9.0000000000000777
Bushnell MC, Ceko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14(7):502–511. https://doi.org/10.1038/nrn3516
Velly AM, Mohit S (2017) Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 87:159–167. https://doi.org/10.1016/j.pnpbp.2017.05.012
Li Y, Wu F, Cheng K, Shen XY, Lao LX (2018) Mechanisms of acupuncture for inflammatory pain. Zhen Ci Yan Jiu 25(8):467–475. https://doi.org/10.13702/j.1000-0607.180196
Zhang R, Lao L, Ren K, Berman BM (2014) Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 120(2):482–503. https://doi.org/10.1097/ALN.0000000000000101
Fu W, Nelson TS, Santos DF, Doolen S, Gutierrez JJP, Ye N, Zhou J, Taylor BK (2019) An NPY Y1 receptor antagonist unmasks latent sensitization and reveals the contribution of protein kinase A and Epac to chronic inflammatory pain. Pain 160(8):1754–1765. https://doi.org/10.1097/j.pain.0000000000001557
Ridiandries A, Tan JTM, Bursill CA (2018) The role of chemokines in wound healing. Int J Mol Sci 19(10):3217. https://doi.org/10.3390/ijms19103217
Pan Z, Li GF, Sun ML, Xie L, Liu D, Zhang Q, Yang XX, Xia S (2019) MicroRNA-1224 splicing circular RNA-Filip1l in an Ago2-dependent manner regulates chronic inflammatory pain via targeting Ubr5. J Neurosci 39(11):2125–2143. https://doi.org/10.1523/JNEUROSCI.1631-18.2018
Zheng J, Jiang YY, Xu LC, Ma LY, Liu FY, Cui S, Cai J, Liao FF (2017) Adult hippocampal neurogenesis along the dorsoventral axis contributes differentially to environmental enrichment combined with voluntary exercise in alleviating chronic inflammatory pain in mice. J Neurosci 37(15):4145–4157. https://doi.org/10.1523/JNEUROSCI.3333-16.2017
Greenberg EN (2012) The consequences of chronic pain. J Pain Palliat Care Pharmacother 26(1):64–67. https://doi.org/10.3109/15360288.2011.650359
Terry MJ, Moeschler SM, Hoelzer BC, Hooten W (2016) Pain catastrophizing and anxiety are associated with heat pain perception in a community sample of adults with chronic pain. Clin J Pain 32:875–881. https://doi.org/10.1097/AJP.0000000000000333
Carroll CP, Brandow AM (2022) Chronic pain: prevalence and management. Hematol Oncol Clin North Am 36(6):1151–1165. https://doi.org/10.1016/j.hoc.2022.06.009
Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K (2005) Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31(4):739–750. https://doi.org/10.1038/sj.npp.1300858
Simons LE, Elman I, Borsook D (2014) Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev 39:61–78. https://doi.org/10.1016/j.neubiorev.2013.12.006
Brent M, Greenwood-Van MB (2009) Role of anxiety in the pathophysiology of irritable bowel syndrome: importance of the amygdala. Front Neurosci 3:47. https://doi.org/10.3389/neuro.21.002.2009
Hsieh JC, Stone-Elander S, Ingvar M (1999) Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci Lett 262(1):61–64. https://doi.org/10.1016/s0304-3940(99)00060-9
Naliboff BD, Chang L, Munakata J, Mayer EA (2000) Towards an integrative model of irritable bowel syndrome. Prog Brain Res 122(122):413–423. https://doi.org/10.1016/s0079-6123(08)62154-8
Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JNP (2001) Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21(24):9896–9903. https://doi.org/10.1523/JNEUROSCI.21-24-09896.2001
Wiech K, Tracey I (2009) The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 47(3):987–994. https://doi.org/10.1016/j.neuroimage.2009.05.059
Middendorp HTV, Lumley MA, Jacobs JWG, Bijlsma JWJ, Geenen R (2010) The effects of anger and sadness on clinical pain reports and experimentally-induced pain thresholds in women with and without fibromyalgia. Arthritis Care Res (Hoboken) 62(10):1370–1376. https://doi.org/10.1002/acr.20230
Hubbard CS, Hong J, Jiang Z, Ebrat B, Suyenobu B, Smith S, Heendeniya N, Naliboff BD (2015) Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterol Motil 27(9):1282–1294. https://doi.org/10.1111/nmo.12622
Bliss TV, Collingridge GL, Kaang BK, Zhuo M (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 17:485–496. https://doi.org/10.1038/nrn.2016.68
Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Yalcin I (2015) The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry 77(3):236–245. https://doi.org/10.1016/j.biopsych.2014.08.004
Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR (2004) Chronic pain patients are impaired on an emotional decision-making task. Pain 108(1-2):129–136. https://doi.org/10.1016/j.pain.2003.12.015
Eisenberger NI, Lieberman MD, Williams KD (2003) Does rejection hurt? An fMRI study of social exclusion. Science 302(5643):290–292. https://doi.org/10.1126/science.1089134
Yoshino A, Okamoto Y, Onoda K, Yoshimura S, Kunisato Y, Demoto Y, Okada G, Yamawaki S (2010) Sadness enhances the experience of pain via neural activation in the anterior cingulate cortex and amygdala: an fMRI study. Neuroimage 50(3):1194–1201. https://doi.org/10.1016/j.neuroimage.2009.11.079
Wise RG, Lujan BJ, Schweinhardt P, Peskett GD, Rogers R, Tracey I (2007) The anxiolytic effects of midazolam during anticipation to pain revealed using fMRI. Magn Reson Imaging 25(6):801–810. https://doi.org/10.1016/j.mri.2007.03.016
Ren D, Li JN, Qiu XT, Wan FP, Wu ZY, Fan BY, Zhang MM, Chen T (2022) Anterior cingulate cortex mediates hyperalgesia and anxiety induced by chronic pancreatitis in rats. Neurosci Bull 38(4):342–358. https://doi.org/10.1007/s12264-021-00800-x
Zhang TT, Guo SS, Wang HY, Jing Q, Yi X, Hu ZH, Yu XR, Xu TL (2022) An anterior cingulate cortex-to-midbrain projection controls chronic itch in mice. Neurosci Bull 39(5):793–807. https://doi.org/10.1007/s12264-022-00996-6
Hanna A, Frangogiannis NG (2020) Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther 34(6):849–863. https://doi.org/10.1007/s10557-020-07071-0
Moser B, Wolf M, Walz A, Loetscher P (2004) Multiple levels of leukocyte migration control. Trends Immunol 25(2):75–84. https://doi.org/10.1016/j.it.2003.12.005
Zhang J, Patel L, Pienta KJJC, Reviews GF (2010) CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 21(1):41–48. https://doi.org/10.1016/j.cytogfr.2009.11.009
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12(2):121–127. https://doi.org/10.1016/s1074-7613(00)80165-x
Sokol CL, Luster AD (2015) The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 7:a016303. https://doi.org/10.1101/cshperspect.a016303
Singh S, Anshita D, Ravichandiran V (2021) MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol 101:107598. https://doi.org/10.1016/j.intimp.2021.107598
Palmqvist C, Wardlaw AJ, Bradding P (2010) Chemokines and their receptors as potential targets for the treatment of asthma. Br J Pharmacol 151(6):725–736. https://doi.org/10.1038/sj.bjp.0707263
Gao YJ, Ji RR (2009) c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 2(1):11–17. https://doi.org/10.2174/1876386300902010011
Liu XJ, Liu T, Chen G, Wang B, Yu XL, Yin C, Ji RR (2016) TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep 6(1):28188. https://doi.org/10.1038/srep28188
Zhang ZJ, Jiang BC, Gao YJ (2017) Chemokines in neuron–glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 74(18):3275–3291. https://doi.org/10.1007/s00018-017-2513-1
Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F (2009) CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 13(3):263–272. https://doi.org/10.1016/j.ejpain.2008.04.017
Muessel MJ, Berman NEJ, Klein RM (2000) Early and specific expression of monocyte chemoattractant protein-1 in the thalamus induced by cortical injury - ScienceDirect. Brain Res 870:211–221. https://doi.org/10.1016/s0006-8993(00)02450-1
Muessel MJ, Klein RM, Wilson AM, Berman NE (2002) Ablation of the chemokine monocyte chemoattractant protein-1 delays retrograde neuronal degeneration, attenuates microglial activation, and alters expression of cell death molecules. Brain Res Mol Brain Res 103(1):12–27. https://doi.org/10.1016/s0169-328x(02)00158-4
Sandhir R, Puri V, Klein RM, Berman NE (2004) Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett 369(1):28–32. https://doi.org/10.1016/j.neulet.2004.07.032
Xie RG, Gao YJ, Park CK, Lu N, Luo C, Wang WT, Wu SX, Ji RR (2017) Spinal CCL2 promotes central sensitization, long-term potentiation, and inflammatory pain via CCR2: further insights into molecular, synaptic, and cellular mechanisms. Neurosci Bull 34:13–21. https://doi.org/10.1007/s12264-017-0106-5
Galasso JM, Liu Y, Szaflarski J, Warren JS, Silverstein F (2000) Monocyte chemoattractant protein-1 is a mediator of acute excitotoxic injury in neonatal rat brain. Neuroscience 101(3):737–744. https://doi.org/10.1016/s0306-4522(00)00399-7
Yao Y, Tsirka SE (2014) Monocyte chemoattractant protein-1 and the blood–brain barrier. Cell Mol Life Sci 71(4):683–697. https://doi.org/10.1007/s00018-013-1459-1
Murugan M, Ravula A, Gandhi A, Vegunta G, Chandra N (2020) Chemokine signaling mediated monocyte infiltration affects anxiety-like behavior following blast injury. Brain Behav Immun 88(6):340–352. https://doi.org/10.1016/j.bbi.2020.03.029
Gabriel AF, Marcus MAE, Walenkamp GHIM, Joosten EA (2009) The CatWalk method: assessment of mechanical allodynia in experimental chronic pain. Behav Brain Res 198(2):477–480. https://doi.org/10.1016/j.bbr.2008.12.018
Xian H, Guo H, Liu YY, Zhang JL, Hu WC, Yu MJ, Zhao R, Xie RG (2023) Peripheral BDNF regulates somatosensory-sympathetic coupling in brachial plexus avulsion-induced neuropathic pain. Neuroscience bulletin. https://doi.org/10.1007/s12264-023-01075-0
Feehan AK, Zadina JE (2019) Morphine immunomodulation prolongs inflammatory and postoperative pain while the novel analgesic ZH853 accelerates recovery and protects against latent sensitization. J Neuroinflammation 16(1):100. https://doi.org/10.1186/s12974-019-1480-x
Li YJ, Zhang K, Sun T, Wang J, Wu YM (2019) Epigenetic suppression of liver X receptor β in anterior cingulate cortex by HDAC5 drives CFA-induced chronic inflammatory pain. J Neuroinflammation 16(1):132. https://doi.org/10.1186/s12974-019-1507-3
Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL (2009) JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 29(13):4096–4108. https://doi.org/10.1523/JNEUROSCI.3623-08.2009
Zhang H, Ma SB, Gao YJ, Xing JL, Xian H, Li ZZ, Shen SN, Wu SX (2020) Spinal CCL2 promotes pain sensitization by rapid enhancement of NMDA-induced currents through the ERK-GluN2B pathway in mouse lamina II neurons. Neurosci Bull 36(11):1344–1354. https://doi.org/10.1007/s12264-020-00557-9
RMW TD (2018) Modeling pain using fMRI: from regions to biomarkers. Neurosci Bull 34(1):208–215. https://doi.org/10.1007/s12264-017-0150-1
Zhuo M (2014) Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 369(1633):20130146. https://doi.org/10.1098/rstb.2013.0146
Labuda CJ, Fuchs PN (2005) Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neuroscience 136(1):311–322. https://doi.org/10.1016/j.neuroscience.2005.07.010
Xu-Hui S, Barthas F, Zhuo M, Victor H, Sylvain A (2018) Hyperactivity of anterior cingulate cortex areas 24a/24b drives chronic pain-induced anxiodepressive-like consequences. J Neurosci 38(12):3102–3115. https://doi.org/10.1523/JNEUROSCI.3195-17.2018
Robbins MT, Deberry J, Ness TJ (2007) Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav 91(5):544–550. https://doi.org/10.1016/j.physbeh.2007.04.009
Kim SS, Wang H, Li XY, Chen T, Zhuo M (2011) Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain 4(1):1–10. https://doi.org/10.1186/1756-6606-4-6
Ivkovi L, Asare Y, Jürgen Bernhagen Dichgans M, Georgakis MK (2022) Pharmacological targeting of the CCL2/CCR2 axis for atheroprotection: a meta-analysis of preclinical studies. Arterioscler Thromb Vasc Biol 42(5):e131–e144. https://doi.org/10.1161/ATVBAHA.122.317492
Gao YJ, Ji RR (2010) Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther 126(1):56–68. https://doi.org/10.1016/j.pharmthera.2010.01.002
Jiang H, Cui H, Wang T, Shimada SG, Sun R, Tan Z, Ma C, Lamotte RH (2019) CCL2/CCR2 signaling elicits itch- and pain-like behavior in a murine model of allergic contact dermatitis. Brain Behav Immun 80:464–473. https://doi.org/10.1016/j.bbi.2019.04.026
Ma SB, Xian H, Wu WB, Ma SY, Xie RG (2020) CCL2 facilitates spinal synaptic transmission and pain via interaction with presynaptic CCR2 in spinal nociceptor terminals. Mol Brain 13(1):161. https://doi.org/10.1186/s13041-020-00701-6
Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS (2009) Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol 215(2):298–307. https://doi.org/10.1016/j.expneurol.2008.10.018
Li Q, Liu S, Li L, Ji X, Wang M, Zhou J (2018) Spinal IL-36γ/IL-36R participates in the maintenance of chronic inflammatory pain through astroglial JNK pathway. Glia 67:438–451. https://doi.org/10.1002/glia.23552
Mao LM (2009) Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci 12(5):602–610. https://doi.org/10.1038/nn.2300
Sanz-Clemente A, Nicoll RA, Roche KW (2013) Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist 19(1):62–75. https://doi.org/10.1177/1073858411435129
Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC (2014) Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345(6196):535–542. https://doi.org/10.1126/science.1253994
Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV (2016) The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 19:220–222. https://doi.org/10.1038/nn.4199
Wang XY, Zhou HR, Wang S, Liu CY, Qin GC, Fu QQ, Zhou JY, Chen L (2018) NR2B-Tyr phosphorylation regulates synaptic plasticity in central sensitization in a chronic migraine rat model. J Headache Pain 19(1):102. https://doi.org/10.1186/s10194-018-0935-2
Kim Y, Cho HY, Ahn YJ, Kim J, Yoon YW (2012) Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain 153(5):1022–1029. https://doi.org/10.1016/j.pain.2012.02.003
Slack S, Battaglia A, Cibert-Goton V, Gavazzi I (2008) EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family kinases during inflammatory hyperalgesia. Neuroscience 156:175–183. https://doi.org/10.1016/j.neuroscience.2008.07.023
Funding
STI 2030-Major Projects (2021ZD0203205) and Natural Science Foundation of China (NSFC) grants (Nos. 82371225, 82171212, 81870867) to Rou-Gang Xie; STI 2030-Major Projects (No. 2021ZD0203104) and Natural Science Foundation of China (NSFC) grants (Nos. 82330036, 32071002) to Ceng Luo.
Author information
Authors and Affiliations
Contributions
RGX, CL, and LYX designed the study. HG, WCH, HX, YXS, YYL, SBM, and KQP performed experiments and analyzed data. RGX, CL, and LYX supervised the project and experiments. RGX, CL, and LYX wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study was performed according to the Helsinki Declaration and approved by the ethical committee of the Fourth Military Medical University. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, H., Hu, Wc., Xian, H. et al. CCL2 Potentiates Inflammation Pain and Related Anxiety-Like Behavior Through NMDA Signaling in Anterior Cingulate Cortex. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03881-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03881-z