Abstract
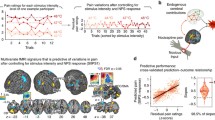
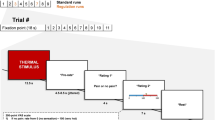
Pain is a subjective and complex phenomenon. Its complexity is related to its heterogeneity: multiple component processes, including sensation, affect, and cognition, contribute to pain experience and reporting. These components are likely to be encoded in distributed brain networks that interact to create pain experience and pain-related decision-making. Therefore, to understand pain, we must identify these networks and build models of these interactions that yield testable predictions about pain-related outcomes. We have developed several such models or ‘signatures’ of pain, by (1) integrating activity across multiple systems, and (2) using pattern-recognition to identify processes related to pain experience. One model, the Neurologic Pain Signature, is sensitive and specific to pain in individuals, involves brain regions that receive nociceptive afferents, and shows little effect of expectation or self-regulation in tests to date. Another, the ‘Stimulus Intensity-Independent Pain Signature’, explains substantial additional variation in trial-to-trial pain reports. It involves many brain regions that do not show increased activity in proportion to noxious stimulus intensity, including medial and lateral prefrontal cortex, nucleus accumbens, and hippocampus. Responses in this system mediate expectancy and perceived control effects in several studies. Overall, this approach provides a pathway to understanding pain by identifying multiple systems that track different aspects of pain. Such componential models can be combined in unique ways on a subject-by-subject basis to explain an individual’s pain experience.


Similar content being viewed by others
References
IASP Task Force on Taxonomy. Part III: Pain Terms, A Current List with Definitions and Notes on Usage. In: Merskey H, Bogduk N (eds). Classification of Chronic Pain. Second Edition. Seattle: IASP Press, 1994:209–214.
Scherer KR. On the nature and function of emotion: A component process approach. In: Scherer KR, Ekman P (Ed.). Approaches to Emotion Psychology Press, 1984: 293–317.
Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A 2005, 102: 12950–12955.
Melzack R. Pain and the neuromatrix in the brain. J Dent Educ 2001, 65: 1378–1382.
Mallam RA. Cultural influences on the patient’s reaction to anxiety and pain. Ghana Nurse 1966, 3: 4–6.
Winston JS, Vlaev I, Seymour B, Chater N, Dolan RJ. Relative valuation of pain in human orbitofrontal cortex. J Neurosci 2014, 34: 14526–14535.
Pincus T, Smeets RJ, Simmonds MJ, Sullivan MJ. The fear avoidance model disentangled: improving the clinical utility of the fear avoidance model. Clin J Pain 2010, 26: 739–746.
Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000, 85: 317–332.
Schwarz N. Self-reports: How the questions shape the answers. Am Psychol 1999, 54: 93–105. doi:10.1037/0003-066X.54.2.93.
Schwartz N, Knauper B, Hippler H, Noelle-Neuman E, Clark F. Rating scales: numeric values may change the meaning of scale labels. Public Opin Q 1991, 55: 570-582. doi:10.1086/269282.
de CWAC, Davies HT, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain 2000, 85: 457–463.
Kahneman D, Riis J. Living, and thinking about it: Two perspectives on life. In: Huppert FA, Baylis N, Keverne B (Ed.). The Science of Well-Being. Oxford Scholarship Online, 2012. doi:10.1093/acprof:oso/9780198567523.003.0011.
Firestone C, Scholl BJ. When do ratings implicate perception vs. judgment? The “overgeneralization test” for top-down effects. Vis Cogn 2016, 23: 1217–1226. doi:10.1080/13506285.2016.1160171.
Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain 2007, 133: 221–228.
Mackenzie N. Phantom limb pain during spinal anaesthesia. Recurrence in amputees. Anaesthesia 1983, 38: 886–887.
Shrestha G, Koirala S. Exacerbation of phantom limb pain following spinal anaesthesia: A case report and review of the literatures. Ain-Shams J Anaesthesiol 2016, 9(2): 309. doi:10.4103/1687-7934.182289.
Xie RG, Gao YJ, Park CK, Lu N, Luo C, Wang WT, et al. Spinal CCL2 promotes central sensitization, long-term potentiation, and inflammatory pain via CCR2: Further insights into molecular, synaptic, and cellular mechanisms. Neurosci Bull 2017. doi:10.1007/s12264-017-0106-5.
Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 2016, 113: E3441–3450.
Liu XJ, Liu T, Chen G, Wang B, Yu XL, Yin C, et al. TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep 2016, 6: 28188.
Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res 2009, 1268: 154–161.
Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 2003, 89: 716–727.
Davis KD. Neuroimaging of pain: what does it tell us? Curr Opin Support Palliat Care 2011, 5: 116–121. .
Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: Is chronicpain a disease? J Pain 2009,10: 1113–1120. .
Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci 2015, 38: 86–95.
Woo CW, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 2017, 20: 365–377.
Flor H, Turk D. Chronic Pain: An Integrated Biobehavioral Approach. Seattle: IASP Press, 2011.
Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry 2014, 171: 395–397.
Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012, 17: 1174–1179.
May A. Neuroimaging: visualising the brain in pain. Neurol Sci 2007, 28 Suppl 2: S101–107.
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005, 9: 463–484.
Melzack R. From the gate to the neuromatrix. Pain 1999, Suppl 6: S121–126.
Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 1999, 82: 1934–1943.
Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2001, 2: 83–91.
Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 2009, 29: 14223–14235.
Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage 2012, 60: 409–418.
Liberati G, Klocker A, Safronova MM, Ferrao Santos S, Ribeiro Vaz JG, Raftopoulos C, et al. Nociceptive local field potentials recorded from the human insula are not specific for nociception. PLoS Biol 2016, 14: e1002345.
Richter M, Eck J, Straube T, Miltner WH, Weiss T. Do words hurt? Brain activation during the processing of pain-related words. Pain 2010, 148: 198–205.
Salomons TV, Iannetti GD, Liang M, Wood JN. The “pain matrix” in pain-free individuals. JAMA Neurol 2016, 73: 755–756.
Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back). Exp Brain Res 2010, 205: 1–12.
Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 2011, 93: 111–124.
Brascher AK, Becker S, Hoeppli ME, Schweinhardt P. Different brain circuitries mediating controllable and uncontrollable pain. J Neurosci 2016, 36: 5013–5025.
Millan MJ. Descending control of pain. Prog Neurobiol 2002, 66: 355–474.
Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci 2004, 25: 613–617.
Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015, 16: 403–418.
Lee M, Manders TR, Eberle SE, Su C, D’Amour J, Yang R, et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci 2015, 35: 5247–5259.
Schwartz N, Miller C, Fields HL. Cortico-Accumbens Regulation of Approach-Avoidance Behavior Is Modified by Experience and Chronic Pain. Cell Rep 2017, 19: 1522–1531.
Hardy SG, Haigler HJ. Prefrontal influences upon the midbrain: a possible route for pain modulation. Brain Res 1985, 339: 285–293.
Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014, 17: 1304–1312.
Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Ann N Y Acad Sci 2013, 1282: 1–11.
Woo CW, Roy M, Buhle JT, Wager TD. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol 2015, 13: e1002036.
Kucyi A, Salomons TV, Davis KD. Cognitive behavioral training reverses the effect of pain exposure on brain network activity. Pain 2016, 157: 1895–1904.
Loggia ML, Berna C, Kim J, Cahalan CM, Martel MO, Gollub RL, et al. The lateral prefrontal cortex mediates the hyperalgesic effects of negative cognitions in chronic pain patients. J Pain 2015, 16: 692–699.
Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006, 120: 297–306.
Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci 2014, 17: 1607–1612.
Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013, 14: 502–511.
Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 2009, 60: 226–242.
Alvarado S, Tajerian M, Millecamps M, Suderman M, Stone LS, Szyf M. Peripheral nerve injury is accompanied by chronic transcriptome-wide changes in the mouse prefrontal cortex. Mol Pain 2013, 9: 21.
Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009, 10: 23–36.
Haynes JD. A Primer on pattern-based approaches to fMRI: principles, pitfalls, and perspectives. Neuron 2015, 87: 257–270.
Haxby J V, Connolly AC, Guntupalli JS. Decoding neural representational spaces using Multivariate Pattern Analysis. Annu Rev Neurosci 2014, 37: 435–456. .
Hanke M, Halchenko YO, Haxby JV, Pollmann S. Statistical learning analysis in neuroscience: aiming for transparency. Front Neurosci 2010, 4: 38.
Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 2009, 45: S199–209.
Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013, 368: 1388–1397.
Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav 2004, 82: 109–114.
Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, Lopez-Sola M, et al. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife 2016, 5.
Ma Y, Wang C, Luo S, Li B, Wager TD, Zhang W, et al. Serotonin transporter polymorphism alters citalopram effects on human pain responses to physical pain. Neuroimage 2016, 135: 186–196.
Woo CW, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, et al. Quantifying cerebral contributions to pain beyond nociception. Nat Commun 2017, 8: 14211.
Reddan MC, Lindquist MA, Wager TD. Effect size estimation in neuroimaging. JAMA Psychiatry 2017, 74: 207–208.
Wager TD, Atlas LY, Botvinick MM, Chang LJ, Coghill RC, Davis KD, et al. Pain in the ACC? Proc Natl Acad Sci U S A 2016, 113: E2474–2475.
Zaki J, Wager TD, Singer T, Keysers C, Gazzola V. The anatomy of suffering: understanding the relationship between nociceptive and empathic pain. Trends Cogn Sci 2016, 20: 249–259.
Ioannidis JPA. Why most published research findings are false. PLoS Med 2005, 2: e124. doi:10.1371/journal.pmed.0020124.
Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017, 18: 115–126.
Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 2009, 4: 274–290.
Lindquist MA, Krishnan A, Lopez-Sola M, Jepma M, Woo CW, Koban L, et al. Group-regularized individual prediction: theory and application to pain. Neuroimage 2017, 145: 274–287.
Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol 2015, 13: e1002180.
Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, et al. Separate neural representations for physical pain and social rejection. Nat Commun 2014, 5: 5380.
Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 2011, 31: 439–452.
Lopez-Sola M, Woo CW, Pujol J, Deus J, Harrison BJ, Monfort J, et al. Towards a neurophysiological signature for fibromyalgia. Pain 2017, 158: 34–47.
Arbabshirani MR, Plis S, Sui J, Calhoun VD. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage 2017, 145: 137–165.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddan, M.C., Wager, T.D. Modeling Pain Using fMRI: From Regions to Biomarkers. Neurosci. Bull. 34, 208–215 (2018). https://doi.org/10.1007/s12264-017-0150-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0150-1