Abstract
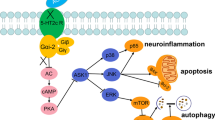
Depression is a common psychological disease with high morbidity and mortality. Recently, the involvement of synaptic plasticity in the pathogenesis of depression has shed light on the direction of developing novel antidepressants. Levomilnacipran is a newly approved medication for the treatment of adult major depressive disorder. However, the detailed mechanisms underlying its antidepressant-like effects have yet to be illuminated. In this study, we aimed to investigate the role of levomilnacipran in regulating synaptic plasticity and explore the possible molecular mechanisms of its antidepressant effects using a rat model of depression induced by lipopolysaccharide (LPS). The results demonstrated that levomilnacipran (30 mg/kg, i.p.) significantly ameliorated depression-like behaviors in rats, alleviated the dysregulation of synaptic plasticity, and suppressed neuroinflammation within hippocampus induced by LPS-treatment. Levomilnacipran increased the expression of postsynaptic dense 95 (PSD-95) and synaptophysin (Syn) and reversed the imbalance between pro- and anti-inflammatory cytokines within hippocampus of depressed rats. Additionally, levomilnacipran elevated expression level of brain-derived neurotrophic factor (BDNF), accompanied by increased tyrosine kinase B (TrkB), phosphorylated phosphatidylinositol 3-kinase (PI3K), phosphorylated protein kinase B (p-Akt), and phosphorylated mammalian target of rapamycin (p-mTOR). Taken together, these results suggest that levomilnacipran may exert antidepressant effects via upregulating BDNF/TrkB mediated PI3K/Akt/mTOR signaling pathway to improve synaptic plasticity. These findings reveal potential mechanisms for the antidepressant effects of levomilnacipran and offer new insights into the treatments for depression.
Graphical Abstract







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- LTP:
-
Long-term potentiation
- LTD:
-
Long-term depression
- AMPAR:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- NMDAR:
-
N-methyl-D-aspartate receptor
- SNRI:
-
Serotonin and norepinephrine reuptake inhibitor
- LPS:
-
Lipopolysaccharide
- SPT:
-
Sucrose preference test
- FST:
-
Forced swim test
- OFT:
-
Open field test
- TEM:
-
Transmission electron microscopy
- ANOVA:
-
Analysis of variance
- PSD-95:
-
Postsynaptic density protein 95
- Syn:
-
Synaptophysin
- BDNF:
-
Brain-derived neurotrophic factor
- TrkB:
-
Tyrosine kinase B
- PI3K:
-
Phosphatidylinositol 3-kinase
- Akt:
-
Protein kinase B
- mTOR:
-
Mammalian target of rapamycin
- CREB:
-
CAMP-responsive element binding protein
- GAPDH:
-
Glyceraldehyde-phosphate dehydrogenase
- IL:
-
Interleukin
References
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Collaborators GBDMD (2022) Global, regional, and national burden of 12 mental disorders in 204 countries and territories 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9:137–150
Castren E (2013) Neuronal network plasticity and recovery from depression. JAMA Psychiat 70:983–989
Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D et al (2011) Harnessing neuroplasticity for clinical applications. Brain 134:1591–1609
Castren E (2005) Is mood chemistry? Nat Rev Neurosci 6:241–246
Frost DO, Tamminga CA, Medoff DR, Caviness V, Innocenti G, Carpenter WT (2004) Neuroplasticity and schizophrenia. Biol Psychiatry 56:540–543
Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS (2013) Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16:69–82
Beneyto M, Meador-Woodruff JH (2008) Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology 33:2175–2186
Eisch AJ, Petrik D (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338:72–75
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818
MacQueen G, Frodl T (2011) The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16:252–264
Jaworska N, Yang XR, Knott V, MacQueen G (2015) A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry 16:448–471
Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, Normann C (2010) Learning as a model for neural plasticity in major depression. Biol Psychiatry 68:544–552
Mago R, Mahajan R, Thase ME (2014) Levomilnacipran: a newly approved drug for treatment of major depressive disorder. Expert Rev Clin Pharmacol 7:137–145
Thase ME, Gommoll C, Chen C, Kramer K, Sambunaris A (2016) Effects of levomilnacipran extended-release on motivation/energy and functioning in adults with major depressive disorder. Int Clin Psychopharmacol 31:332–340
Wagner G, Schultes MT, Titscher V, Teufer B, Klerings I, Gartlehner G (2018) Efficacy and safety of levomilnacipran, vilazodone and vortioxetine compared with other second-generation antidepressants for major depressive disorder in adults: a systematic review and network meta-analysis. J Affect Disord 228:1–12
Montgomery SA, Mansuy L, Ruth A, Bose A, Li H, Li D (2013) Efficacy and safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry 74:363–369
Krause-Sorio B, Kilpatrick L, Siddarth P, Ercoli L, Laird KT, Aguilar-Faustino Y, Milillo MM, Narr KL, Lavretsky H (2020) Cortical thickness increases with levomilnacipran treatment in a pilot randomised double-blind placebo-controlled trial in late-life depression. Psychogeriatrics 20:140–148
Bian H, Wang G, Huang J, Liang L, Zheng Y, Wei Y, Wang H, Xiao L, Wang H (2020) Dihydrolipoic acid protects against lipopolysaccharide-induced behavioral deficits and neuroinflammation via regulation of Nrf2/HO-1/NLRP3 signaling in rat. J Neuroinflammation 17:166
Auclair AL, Martel JC, Assie MB, Bardin L, Heusler P, Cussac D, Marien M, Newman-Tancredi A, O’Connor JA, Depoortere R (2013) Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology 70:338–347
Naegeli KJ, O’Connor JA, Banerjee P, Morilak DA (2013) Effects of milnacipran on cognitive flexibility following chronic stress in rats. Eur J Pharmacol 703:62–66
Matsumoto M, Tachibana K, Togashi H, Tahara K, Kojima T, Yamaguchi T, Yoshioka M (2005) Chronic treatment with milnacipran reverses the impairment of synaptic plasticity induced by conditioned fear stress. Psychopharmacology 179:606–612
Lan T, Wu Y, Zhang Y, Li S, Zhu Z, Wang L, Mao X, Li Y, Fan C, Wang W, Yu SY (2022) Agomelatine rescues lipopolysaccharide-induced neural injury and depression-like behaviors via suppression of the Galphai-2-PKA-ASK1 signaling pathway. J Neuroinflammation 19:117
Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS (2007) A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry 61:661–670
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
Keers R, Uher R (2012) Gene-environment interaction in major depression and antidepressant treatment response. Curr Psychiatry Rep 14:129–137
Bruno A, Morabito P, Spina E, Muscatello MR (2016) The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuropharmacol 14:191–199
Lee SM, Dong TS, Krause-Sorio B, Siddarth P, Milillo MM, Lagishetty V, Datta T, Aguilar-Faustino Y, Jacobs JP, Lavretsky H (2022) The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: a prospective pilot study. Int Psychogeriatr 34:33–45
Naguy A (2021) Levomilnacipran for negative symptom domain schizophrenia. Prim Care Companion CNS Disord 23
Rizvi SM, Shaikh S, Khan M, Biswas D, Hameed N, Shakil S (2014) Fetzima (levomilnacipran), a drug for major depressive disorder as a dual inhibitor for human serotonin transporters and beta-site amyloid precursor protein cleaving enzyme-1. CNS Neurol Disord Drug Targets 13:1427–1431
Masi G, Brovedani P (2011) The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression. CNS Drugs 25:913–931
MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R (2008) Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry 64:880–883
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56:640–650
Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T et al (1991) The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 65:895–903
Smith MA, Makino S, Kvetnansky R, Post RM (1995) Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15:1768–1777
Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RC, Elzinga BM (2011) Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 16:1088–1095
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23
Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY (2005) Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25:11288–11299
Xu F, Na L, Li Y, Chen L (2020) Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10:54
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249
Tao X, West AE, Chen WG, Corfas G, Greenberg ME (2002) A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron 33:383–395
Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A (2012) Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 109:E197-205
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Maes M (1994) Cytokines in major depression. Biol Psychiatry 36:498–499
Acknowledgements
We thank Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work.
Funding
This study was supported by grants to Shu Yan Yu from the National Natural Science Foundation of China (82071513; 82271566) and the Natural Science Foundation of Shandong Province of China (ZR2021MH151; ZR2020MH152).
Author information
Authors and Affiliations
Contributions
Conceptualization: SYY, YW, and TL; methodology: YW, ZZ, and TL; investigation: YW, TL, SL, YL, and CW; funding acquisition: SYY; writing: YW, TL, and SYY; supervision: YF, XM, and SYY.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The Ethics Committee at Shandong University Animal Care and Use Committee (Jinan, China) approved the protocols of this study. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). All authors declare that they consent to participate and approve for the publication of this article.
Consent for Publication
All authors declared to consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
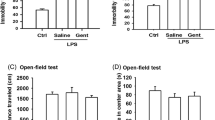
1. Levomilnacipran ameliorated anxiety- and depression-like behaviors in rats.
2. Levomilnacipran alleviated LPS-induced dysregulation of synaptic plasticity.
3. Levomilnacipran ameliorated LPS-induced neuroinflammation.
4. Levomilnacipran activated the BDNF/TrkB mediated PI3K/Akt/mTOR pathway.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Zhu, Z., Lan, T. et al. Levomilnacipran Improves Lipopolysaccharide-Induced Dysregulation of Synaptic Plasticity and Depression-Like Behaviors via Activating BDNF/TrkB Mediated PI3K/Akt/mTOR Signaling Pathway. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03832-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03832-8