Abstract
Neurodegenerative diseases (NDDs) and neuropsychiatric disorders (NPDs) are two common causes of death in elderly people, which includes progressive neuronal cell death and behavioral changes. NDDs include Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and motor neuron disease, characterized by cognitive defects and memory impairment, whereas NPDs include depression, seizures, migraine headaches, eating disorders, addictions, palsies, major depressive disorders, anxiety, and schizophrenia, characterized by behavioral changes. Mounting evidence demonstrated that NDDs and NPDs share an overlapping mechanism, which includes post-translational modifications, the microbiota–gut–brain axis, and signaling events. Mounting evidence demonstrated that various drug molecules, namely, natural compounds, repurposed drugs, multitarget directed ligands, and RNAs, have been potentially implemented as therapeutic agents against NDDs and NPDs. Herein, we highlighted the overlapping mechanism, the role of anxiety/stress-releasing factors, cytosol-to-nucleus signaling, and the microbiota–gut–brain axis in the pathophysiology of NDDs and NPDs. We summarize the therapeutic application of natural compounds, repurposed drugs, and multitarget-directed ligands as therapeutic agents. Lastly, we briefly described the application of RNA interferences as therapeutic agents in the pathogenesis of NDDs and NPDs.
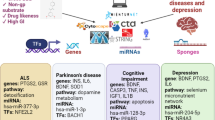
Graphical Abstract

Neurodegenerative diseases and neuropsychiatric diseases both share a common signaling molecule and molecular phenomenon, namely, pro-inflammatory cytokines, γCaMKII and MAPK/ERK, chemokine receptors, BBB permeability, and the gut–microbiota–brain axis. Studies have demonstrated that any alterations in the signaling mentioned above molecules and molecular phenomena lead to the pathophysiology of neurodegenerative diseases, namely, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, and neuropsychiatric disorders, such as bipolar disorder, schizophrenia, depression, anxiety, autism spectrum disorder, and post-traumatic stress disorder.




Similar content being viewed by others
Data Availability
This is not applicable.
Abbreviations
- NDDs:
-
Neurodegenerative diseases
- NPDs:
-
Neuropsychiatric disorders
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington's disease
- ALS:
-
Amyotrophic lateral sclerosis
- CNS:
-
Central nervous system
- miRNA:
-
MicroRNA
- ncRNA:
-
Non-coding RNAs
- PTM:
-
Post-translational modifications
- HATs:
-
Histone acetyltransferase
- HDACs:
-
Histone deacetylases
- DNMTs:
-
DNA methyltransferases
- TSPO:
-
The 18-kDa translocator protein
- mTOR:
-
Mammalian target of rapamycin
- Aβ:
-
β-Amyloid
- CRF:
-
Corticotropin-releasing factor
- TNFα:
-
Tumor necrosis factor alpha
- MCP-1:
-
Monocyte chemoattractant protein-1
- CCL2:
-
Chemokine (C-C motif) ligand 2
- OXTR:
-
Oxytocin receptor gene
- TCM:
-
Traditional Chinese medicine
- SSRIs:
-
Selective serotonin reuptake inhibitors
- IL-1α:
-
Interleukin 1 alpha
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin 6
- BDNF:
-
Brain-derived neurotropic factor
- ASD:
-
Autism spectrum disorder
- TLR4:
-
Toll-like receptor 4
- MyD88:
-
Myeloid differentiation primary response 88
- NF-κB:
-
Nuclear factor kappa B
- γCaMKII:
-
γ-Ca2+/calmodulin-dependent protein kinase II
- CREB:
-
cAMP response element-binding protein
- GluN2B:
-
Glutamate receptor subunit epsilon-2
- PSD95:
-
Postsynaptic density protein 95
- PKA:
-
Protein kinase A
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Serine/threonine protein kinase
- ROS:
-
Reactive oxygen species
- PET:
-
Positron emission tomography
- c/EBPβ:
-
CCAAT/enhancer-binding protein beta
- UBR5:
-
Ubiquitin protein ligase E3 component N-Recognin 5
- iPSCs:
-
Induced pluripotent stem cells
- ASB17:
-
Ankyrin repeat, and SOCS box protein 17
- TDP-43:
-
TAR DNA-binding protein 43
- LTN1:
-
Listerin1
- CUL3:
-
Cullin3
- MeCP2:
-
Methyl CpG binding protein 2
- CAM:
-
Complementary and alternative medicine
- MTDL:
-
Multitarget directed ligand
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- GLP-1:
-
Glucagon-like peptide-1
- PPAR-γ:
-
Peroxisome proliferator- activated receptor gamma
- SDAM:
-
Serotonin–dopamine activity modulators
- TLR-2:
-
Toll-like receptor-2
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- CDK5:
-
Cyclin-dependent kinase 5
- TGF-β:
-
Transforming growth factor beta
- GRM4:
-
Glutamate receptor 4, metabotropic
- MALAT1:
-
Metastasis-related lung adenocarcinoma transcript 1
- NEAT1:
-
Nuclear enriched abundant transcript 1
- DISC2:
-
Disrupted in schizophrenia 2
- BDNF-AS:
-
Brain-derived neurotrophic factor antisense
References
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9:a028035. https://doi.org/10.1101/CSHPERSPECT.A028035
Scheltens P, De Strooper B, Kivipelto M et al (2021) Alzheimer’s disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Matej R, Tesar A, Rusina R (2019) Alzheimer’s disease and other neurodegenerative dementias in comorbidity: a clinical and neuropathological overview. Clin Biochem 73:26–31. https://doi.org/10.1016/J.CLINBIOCHEM.2019.08.005
Uwishema O, Onyeaka H, Badri R et al (2022) The understanding of Parkinson’s disease through genetics and new therapies. Brain Behav 12:e2577. https://doi.org/10.1002/BRB3.2577
Tabrizi SJ, Estevez-Fraga C, van Roon-Mom WMC et al (2022) Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol 21:645–658. https://doi.org/10.1016/S1474-4422(22)00121-1
McAllister B, Gusella JF, Landwehrmeyer GB et al (2021) Timing and impact of psychiatric, cognitive, and motor abnormalities in Huntington disease. Neurology 96:e2395–e2406. https://doi.org/10.1212/WNL.0000000000011893
Mead RJ, Shan N, Reiser HJ et al (2022) Amyotrophic lateral sclerosis: a neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov 223(22):185–212. https://doi.org/10.1038/s41573-022-00612-2
Bray NJ, O’Donovan MC (2018) The genetics of neuropsychiatric disorders. Brain Neurosci Adv 2:239821281879927. https://doi.org/10.1177/2398212818799271
Stilo SA, Murray RM (2019) Non-genetic factors in schizophrenia. Curr Psychiatry Rep 21:1–10. https://doi.org/10.1007/S11920-019-1091-3/FIGURES/1
Spark DL, Fornito A, Langmead CJ, Stewart GD (2022) Beyond antipsychotics: a twenty-first century update for preclinical development of schizophrenia therapeutics. Transl Psychiatry 121(12):1–11. https://doi.org/10.1038/s41398-022-01904-2
Otte C, Gold SM, Penninx BW et al (2016) Major depressive disorder. Nat Rev Dis Prim 21(2):1–20. https://doi.org/10.1038/nrdp.2016.65
McIntyre RS, Berk M, Brietzke E et al (2020) Bipolar disorders. Lancet 396:1841–1856. https://doi.org/10.1016/S0140-6736(20)31544-0
Shirvani Farsani Z, Zahirodin A, Ghaderian SMH et al (2020) The role of long non-coding RNA MALAT1 in patients with bipolar disorder. Metab Brain Dis 35:1077–1083. https://doi.org/10.1007/s11011-020-00580-9
Sokoloff P, Diaz J, Foll BL et al (2006) The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord-Drug Targets Formerly Current Drug Targets-CNS Neurol Disord 5(1):25–43. https://doi.org/10.2174/187152706775535687
Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 86(8):413–426. https://doi.org/10.1038/nrn2153
Pasquini S, Contri C, Merighi S et al (2022) Adenosine receptors in neuropsychiatric disorders: fine regulators of neurotransmission and potential therapeutic targets. Int J Mol Sci 1219(23):1219. https://doi.org/10.3390/IJMS23031219
Pourhamzeh M, Moravej FG, Arabi M et al (2021) The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol 426(42):1671–1692. https://doi.org/10.1007/S10571-021-01064-9
Athar T, Al Balushi K, Khan SA (2021) Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep 48:5629–5645. https://doi.org/10.1007/S11033-021-06512-9/FIGURES/8
Gouda NA, Elkamhawy A, Cho J (2022) Emerging therapeutic strategies for Parkinson’s disease and future prospects: a 2021 update. Biomed 371(10):371. https://doi.org/10.3390/BIOMEDICINES10020371
Lovedhi Aggarwal MFACM (2011) Therapeutic management of late-stage Parkinson’s disease: review of the Movement Disorder Society’s guidelines. Ann Long-Term Care 19
Kim A, Lalonde K, Truesdell A et al (2021) New avenues for the treatment of Huntington’s disease. Int J Mol Sci 8363(22):8363. https://doi.org/10.3390/IJMS22168363
Nowicka N, Juranek J, Juranek JK, Wojtkiewicz J (2019) Risk factors and emerging therapies in amyotrophic lateral sclerosis. Int J Mol Sci 2616(20):2616. https://doi.org/10.3390/IJMS20112616
Yang JH, Rempe T, Whitmire N et al (2022) Therapeutic advances in multiple sclerosis. Front Neurol 13:824926. https://doi.org/10.3389/FNEUR.2022.824926/BIBTEX
De Angelis F, John NA, Brownlee WJ (2018) Disease-modifying therapies for multiple sclerosis. BMJ 363. https://doi.org/10.1136/BMJ.K4674
Shim KH, Sharma N, An SSA (2022) Prion therapeutics: lessons from the past. Prion 16:265. https://doi.org/10.1080/19336896.2022.2153551
Mathew SJ, Price RB, Charney DS (2008) Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet Part C Semin Med Genet 148C:89–98. https://doi.org/10.1002/AJMG.C.30172
Bandelow B, Michaelis S, Wedekind D (2022) Treatment of anxiety disorders. Dialogues Clin Neurosci 19:93–107. 10.31887/DCNS.2017.19.2/BBANDELOW
Voineskos D, Daskalakis ZJ, Blumberger DM (2020) Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat 16:221–234. https://doi.org/10.2147/NDT.S198774
Guery D, Rheims S (2021) Clinical management of drug resistant epilepsy: a review on current strategies. Neuropsychiatr Dis Treat 17:2229–2242. https://doi.org/10.2147/NDT.S256699
Pereira AC, Oliveira J, Silva S et al (2021) Inflammation in bipolar disorder (BD): identification of new therapeutic targets. Pharmacol Res 163:105325. https://doi.org/10.1016/J.PHRS.2020.105325
Ginsberg SD, Elarova I, Ruben M et al (2004) Single-cell gene expression analysis: implications for neurodegenerative and neuropsychiatric disorders. Neurochem Res 296(29):1053–1064. https://doi.org/10.1023/B:NERE.0000023593.77052.F7
Cuttler K, Hassan M, Carr J et al (2021) Emerging evidence implicating a role for neurexins in neurodegenerative and neuropsychiatric disorders. Open Biol 11. https://doi.org/10.1098/RSOB.210091
Rudrapal M, Khairnar SJ, Jadhav AG (2020) Drug repurposing (DR): an emerging approach in drug discovery. Drug Repurposing - Hypothesis, Mol Asp Ther Appl. https://doi.org/10.5772/INTECHOPEN.93193
Wen H, Jung H, Li X (2015) Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J 17:1327. https://doi.org/10.1208/S12248-015-9814-9
Vafadari B (2021) Stress and the role of the gut–brain axis in the pathogenesis of schizophrenia: a literature review. Int J Mol Sci 22. https://doi.org/10.3390/IJMS22189747
Michalska P, León R (2020) When it comes to an end: oxidative stress crosstalk with protein aggregation and neuroinflammation induce neurodegeneration. Antioxidants 740(9):740. https://doi.org/10.3390/ANTIOX9080740
Gimson A, Schlosser M, Huntley JD, Marchant NL (2018) Support for midlife anxiety diagnosis as an independent risk factor for dementia: a systematic review. BMJ Open 8:e019399. https://doi.org/10.1136/BMJOPEN-2017-019399
Cobos SN, Bennett SA, Torrente MP (2019) The impact of histone post-translational modifications in neurodegenerative diseases. Biochim Biophys Acta - Mol Basis Dis 1865:1982–1991. https://doi.org/10.1016/J.BBADIS.2018.10.019
Polajnar M, Žerovnik E (2014) Impaired autophagy: a link between neurodegenerative and neuropsychiatric diseases. J Cell Mol Med 18:1705–1711. https://doi.org/10.1111/jcmm.12349
Ryskalin L, Limanaqi F, Frati A et al (2018) mTOR-related brain dysfunctions in neuropsychiatric disorders. Int J Mol Sci 19
Young FB, Butland SL, Sanders SS et al (2012) Putting proteins in their place: palmitoylation in Huntington disease and other neuropsychiatric diseases. Prog Neurobiol 97:220–238
Rajkhowa B, Mehan S, Sethi P, Prajapati A (2022) Activation of SIRT-1 signalling in the prevention of bipolar disorder and related neurocomplications: target activators and influences on neurological dysfunctions. Neurotox Res 40(2):670–686
Inestrosa NC, Montecinos-Oliva C, Fuenzalida M (2012) Wnt signaling: role in Alzheimer disease and schizophrenia. J Neuroimmune Pharmacol 7:788–807
Siafarikas N, Alnæs D, Monereo-Sanchez J et al (2021) Neuropsychiatric symptoms and brain morphology in patients with mild cognitive impairment and Alzheimer’s disease with dementia. Int Psychogeriatrics 33:1217–1228. https://doi.org/10.1017/S1041610221000934
Wenqi L, Duan J, Zhang W et al (2021) Relationship between neuropsychiatric symptoms and cognitive functions in patients with cognitive impairment. Psychogeriatrics 21:773–782. https://doi.org/10.1111/psyg.12738
Correia AS, Cardoso A, Vale N (2021) Highlighting immune system and stress in major depressive disorder, Parkinson’s, and Alzheimer’s diseases, with a connection with serotonin. Int J Mol Sci 22
Ross JA, Gliebus G, Van Bockstaele EJ (2018) Stress induced neural reorganization: a conceptual framework linking depression and Alzheimer’s disease. Prog Neuro-Psychopharmacology Biol Psychiatry 85:136–151
Moret C, Briley M (2011) The importance of norepinephrine in depression. Neuropsychiatr Dis Treat 7:9. https://doi.org/10.2147/NDT.S19619
Sivolap YP (2021) Serotonin-norepinephrine reuptake inhibitors in psychiatry and neurology. Zhurnal Nevrol i Psikhiatrii Im SS Korsakova 121:141–146. https://doi.org/10.17116/JNEVRO2021121081141
Licinio J, Gold PW (1991) 4 Role of corticotrophin releasing hormone 41 in depressive illness. Baillieres Clin Endocrinol Metab 5:51–58. https://doi.org/10.1016/S0950-351X(05)80096-5
Shekhar A, Truitt W, Rainnie D, Sajdyk T (2009) Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 8:209–219. https://doi.org/10.1080/10253890500504557
Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A et al (2022) Multiple aspects of inappropriate action of renin–angiotensin, vasopressin, and oxytocin systems in neuropsychiatric and neurodegenerative diseases. J Clin Med 908(11):908. https://doi.org/10.3390/JCM11040908
Vashadze SV (2015) Serotonin and depression. BMJ 350:19–21. https://doi.org/10.1136/BMJ.H1771
Kucwaj-Brysz K, Baltrukevich H, Czarnota K, Handzlik J (2021) Chemical update on the potential for serotonin 5-HT6 and 5-HT7 receptor agents in the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 49:128275. https://doi.org/10.1016/J.BMCL.2021.128275
Cacabelos R, Carrera I, Martínez O et al (2021) Influence of dopamine, noradrenaline, and serotonin transporters on the pharmacogenetics of Atremorine in Parkinson’s disease. Drug Dev Res 82:695–706. https://doi.org/10.1002/DDR.21784
Nguyen C, Mondoloni S, Le Borgne T et al (2021) Nicotine inhibits the VTA-to-amygdala dopamine pathway to promote anxiety. Neuron 109:2604–2615.e9. https://doi.org/10.1016/J.NEURON.2021.06.013
Ressler KJ, Nemeroff CB (1999) Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry 46:1219–1233. https://doi.org/10.1016/S0006-3223(99)00127-4
Hakamata Y, Mizukami S, Izawa S et al (2022) Implicit and explicit emotional memory recall in anxiety and depression: role of basolateral amygdala and cortisol-norepinephrine interaction. Psychoneuroendocrinology 136:105598. https://doi.org/10.1016/J.PSYNEUEN.2021.105598
Inoshita T, Hirano T (2021) Norepinephrine facilitates induction of long-term depression through β-adrenergic receptor at parallel fiber-to-Purkinje cell synapses in the flocculus. Neuroscience 462:141–150. https://doi.org/10.1016/J.NEUROSCIENCE.2020.05.037
Hirano T, Inoshita T (2021) Contribution of norepinephrine to cerebellar long-term depression and motor learning. Contemp Clin Neurosci 337–348. https://doi.org/10.1007/978-3-030-75817-2_16
Goddard AW, Ball SG, Martinez J et al (2010) Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 27:339–350. https://doi.org/10.1002/DA.20642
Mausbach BT, Ancoli-Israel S, Von Känel R et al (2006) Sleep disturbance, norepinephrine, and D-dimer are all related in elderly care-givers of people with Alzheimer disease. Sleep 29:1347–1352. https://doi.org/10.1093/SLEEP/29.10.1347
Hoffmeister JD, Kelm-Nelson CA, Ciucci MR (2021) Quantification of brainstem norepinephrine relative to vocal impairment and anxiety in the Pink1-/- rat model of Parkinson disease. Behav Brain Res 414:113514. https://doi.org/10.1016/J.BBR.2021.113514
Hoffmeister JD, Kelm-Nelson CA, Ciucci MR (2022) Manipulation of vocal communication and anxiety through pharmacologic modulation of norepinephrine in the Pink1-/- rat model of Parkinson disease. Behav Brain Res 418:113642. https://doi.org/10.1016/J.BBR.2021.113642
Wiersielis K, Yasrebi A, Roepke T (2021) Intermittent fasting alters cognition, anxiety-like behavior, and hippocampal norepinephrine content in aged mice. FASEB J 35. https://doi.org/10.1096/FASEBJ.2021.35.S1.04428
Taché Y (2015) Corticotrophin-releasing factor 1 activation in the central amygdale and visceral hyperalgesia. Neurogastroenterol Motil 27:1–6. https://doi.org/10.1111/NMO.12495
Chatterjee B, Jain R, Chawla N, Raghav R (2022) Serum corticotrophin releasing factor (CRF) and its correlation with stress and craving in detoxified opioid-dependent subjects. Asian J Psychiatr 68:102964. https://doi.org/10.1016/J.AJP.2021.102964
Knapp DJ, Whitman BA, Wills TA et al (2011) Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain Behav Immun 25:S146–S154. https://doi.org/10.1016/J.BBI.2011.02.018
Sher L, Oquendo MA, Burke AK et al (2013) Combined dexamethasone suppression–corticotrophin-releasing hormone stimulation test in medication-free major depression and healthy volunteers. J Affect Disord 151:1108–1112. https://doi.org/10.1016/J.JAD.2013.06.049
Bhutada P, Mundhada Y, Bansod K et al (2010) Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog Neuro-Psychopharmacology Biol Psychiatry 34:955–960. https://doi.org/10.1016/J.PNPBP.2010.04.025
Ardianto C, Budiatin AS, Sumartha INB et al (2021) Resveratrol ameliorates physical and psychological stress-induced depressive-like behavior. J Basic Clin Physiol Pharmacol 32:335–340. https://doi.org/10.1515/JBCPP-2020-0437/MACHINEREADABLECITATION/RIS
von Bardeleben U, Holsboer F (1989) Cortisol response to a combined dexamethasone-human corticotrophin-releasing hormone challenge in patients with depression. J Neuroendocrinol 1:485–488. https://doi.org/10.1111/J.1365-2826.1989.TB00150.X
Peng B, Xu Q, Liu J et al (2021) Corticosterone attenuates reward-seeking behavior and increases anxiety via D2 receptor signaling in ventral tegmental area dopamine neurons. J Neurosci 41:1566–1581. https://doi.org/10.1523/JNEUROSCI.2533-20.2020
Abuirmeileh A, Lever R, Kingsbury AE et al (2007) The corticotrophin-releasing factor-like peptide urocortin reverses key deficits in two rodent models of Parkinson’s disease. Eur J Neurosci 26:417–423. https://doi.org/10.1111/J.1460-9568.2007.05653.X
Borroto-Escuela DO, Ambrogini P, Chruścicka B et al (2021) The role of central serotonin neurons and 5-HT heteroreceptor complexes in the pathophysiology of depression: a historical perspective and future prospects. Int J Mol Sci 1927(22):1927. https://doi.org/10.3390/IJMS22041927
Dunlop BW, Nemeroff CB (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. https://doi.org/10.1001/ARCHPSYC.64.3.327
Karayol R, Medrihan L, Warner-Schmidt JL et al (2021) Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Mol Psychiatry 266(26):2334–2349. https://doi.org/10.1038/s41380-020-00994-y
Jellen LC, Lewis MM, Du G et al (2021) Low plasma serotonin linked to higher nigral iron in Parkinson’s disease. Sci Rep 111(11):1–12. https://doi.org/10.1038/s41598-021-03700-2
Feng ST, Le WX, Wang YT et al (2021) Efficacy of traditional chinese medicine combined with selective serotonin reuptake inhibitors on the treatment for Parkinson’s disease with depression: a systematic review and meta-analysis. Am J Chin Med 49:627–643. https://doi.org/10.1142/S0192415X21500282
Ahmad MH, Moshahid Alam Rizvi M, Fatima M, Mondal AC (2021) Impact of NGF signaling on neuroplasticity during depression: insights in neuroplasticity-dependent therapeutic approaches. Neurosci Depress Genet Cell Biol Neurol Behav Diet 341–350. https://doi.org/10.1016/B978-0-12-817935-2.00032-5
Tuszynski MH, Yang JH, Barba D et al (2015) Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease. JAMA Neurol 72:1139–1147. https://doi.org/10.1001/JAMANEUROL.2015.1807
Xu D, Wu D, Qin M et al (2019) Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv Mater 31:1900727. https://doi.org/10.1002/ADMA.201900727
Kopach O, Pavlov AM, Sindeeva OA et al (2020) Biodegradable microcapsules loaded with nerve growth factor enable neurite guidance and synapse formation. Pharmaceutics 25(13):25. https://doi.org/10.3390/PHARMACEUTICS13010025
Li R, Li D, Wu C et al (2020) Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 10:1649. https://doi.org/10.7150/THNO.40919
Mondal AC, Fatima M (2019) Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. Int J Neurosci 129:283–296. https://doi.org/10.1080/00207454.2018.1527328
Peng Z, Zhang C, Yan L et al (2020) EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippcampal apoptosis signaling in a chronic stress-induced rat model of depression. Int J Mol Sci 1769(21):1769. https://doi.org/10.3390/IJMS21051769
Chen YW, Lin PY, Tu KY et al (2015) Significantly lower nerve growth factor levels in patients with major depressive disorder than in healthy subjects: a meta-analysis and systematic review. Neuropsychiatr Dis Treat 11:925. https://doi.org/10.2147/NDT.S81432
Pedrotti Moreira F, Cardoso TC, Mondin TC et al (2019) Serum level of nerve growth factor is a potential biomarker of conversion to bipolar disorder in women with major depressive disorder. Psychiatry Clin Neurosci 73:590–593. https://doi.org/10.1111/PCN.12896
Petrella C, Farioli-Vecchioli S, Cisale GY et al (2021) A healthy gut for a healthy brain: preclinical, clinical and regulatory aspects. Curr Neuropharmacol 19:610–628. https://doi.org/10.2174/1570159X18666200730111528
Konjevod M, Nikolac Perkovic M, Sáiz J et al (2021) Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J Pharm Biomed Anal 194:113681. https://doi.org/10.1016/J.JPBA.2020.113681
Lucidi L, Pettorruso M, Vellante F et al (2021) Gut microbiota and bipolar disorder: an overview on a novel biomarker for diagnosis and treatment. Int J Mol Sci 22. https://doi.org/10.3390/IJMS22073723
Kelly JR, Clarke G, Cryan JF, Dinan TG (2016) Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol 26:366–372. https://doi.org/10.1016/J.ANNEPIDEM.2016.02.008
Wu S, Bekhit AEDA, Wu Q et al (2021) Bioactive peptides and gut microbiota: candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci Technol 108:164–176. https://doi.org/10.1016/J.TIFS.2020.12.019
Agirman G, Yu KB, Hsiao EY (2021) Signaling inflammation across the gut-brain axis. Science 374:1087–1092. https://doi.org/10.1126/SCIENCE.ABI6087/ASSET/810B821D-6005-435E-8F2D-953546DB4BF4/ASSETS/IMAGES/LARGE/SCIENCE.ABI6087-F2.JPG
Morais LH, Schreiber HL, Mazmanian SK (2020) The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol 194(19):241–255. https://doi.org/10.1038/s41579-020-00460-0
Sayana P, Colpo GD, Simões LR et al (2017) A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res 92:160–182. https://doi.org/10.1016/J.JPSYCHIRES.2017.03.018
Naaldijk YM, Bittencourt MC, Sack U, Ulrich H (2016) Kinins and microglial responses in bipolar disorder: a neuroinflammation hypothesis. Biol Chem 397:283–296. https://doi.org/10.1515/HSZ-2015-0257/ASSET/GRAPHIC/J_HSZ-2015-0257_FIG_002.JPG
Gondalia S, Parkinson L, Stough C, Scholey A (2019) Gut microbiota and bipolar disorder: a review of mechanisms and potential targets for adjunctive therapy. Psychopharmacol 2365(236):1433–1443. https://doi.org/10.1007/S00213-019-05248-6
Painold A, Mörkl S, Kashofer K et al (2019) A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord 21:40–49. https://doi.org/10.1111/BDI.12682
Kunugi H (2021) Gut microbiota and pathophysiology of depressive disorder. Ann Nutr Metab 77:11–20. https://doi.org/10.1159/000518274
Martin CR, Osadchiy V, Kalani A, Mayer EA (2018) The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6:133. https://doi.org/10.1016/J.JCMGH.2018.04.003
Iannone LF, Preda A, Blottière HM et al (2019) Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev Neurother 19:1037–1050. https://doi.org/10.1080/14737175.2019.1638763
Deng W, Li F, Ke H et al (2022) Effect of metformin in autistic BTBR T+Itpr3tf/J Mice administered a high-fat diet. Brain Res Bull 183:172–183. https://doi.org/10.1016/J.BRAINRESBULL.2022.02.021
Messias EL, Chen CY, Eaton WW (2007) Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am 30:323. https://doi.org/10.1016/J.PSC.2007.04.007
Zhuang ZQ, Shen LL, Li WW et al (2018) Gut microbiota is altered in patients with Alzheimer’s Disease. J Alzheimer’s Dis 63:1337–1346. https://doi.org/10.3233/JAD-180176
Ling Z, Zhu M, Liu X et al (2021) Fecal fungal dysbiosis in Chinese patients with Alzheimer’s disease. Front cell Dev Biol 8. https://doi.org/10.3389/FCELL.2020.631460
Vogt NM, Kerby RL, Dill-McFarland KA et al (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Reports 71(7):1–11. https://doi.org/10.1038/s41598-017-13601-y
Harach T, Marungruang N, Duthilleul N et al (2017) Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Nat Publ Gr. https://doi.org/10.1038/srep41802
Fujii Y, Nguyen TTT, Fujimura Y et al (2019) Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. OUP 83:2144–2152. https://doi.org/10.1080/09168451.2019.1644149
Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7:268242. https://doi.org/10.3389/FCIMB.2017.00318/BIBTEX
Arentsen T, Qian Y, Gkotzis S et al (2016) The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry 222(22):257–266. https://doi.org/10.1038/mp.2016.182
Cao Y, Mezzenga R (2019) Food protein amyloid fibrils: origin, structure, formation, characterization, applications and health implications. Adv Colloid Interface Sci 269:334–356. https://doi.org/10.1016/J.CIS.2019.05.002
Singh RK, Chang HW, Yan D et al (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. https://doi.org/10.1186/S12967-017-1175-Y
Rietdijk CD, Perez-Pardo P, Garssen J et al (2017) Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol 8:37. https://doi.org/10.3389/FNEUR.2017.00037/BIBTEX
Hopfner F, Künstner A, Müller SH et al (2017) Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667:41–45. https://doi.org/10.1016/J.BRAINRES.2017.04.019
Scheperjans F, Aho V, Pereira PAB et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. https://doi.org/10.1002/MDS.26069
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139:216–231. https://doi.org/10.1111/JNC.13731
Hernández-Romero MDC, Argüelles S, Villarán RF et al (2008) Simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intranigral injection of lipopolysaccharide. J Neurochem 105:445–459. https://doi.org/10.1111/J.1471-4159.2007.05148.X
Manole E, Dumitrescu L, Niculiţe CM, Popescu BO Potential roles of functional bacterial amyloid proteins, bacterial biosurfactants and other putative gut microbiota products in the etiopathogeny of Parkinson’s Disease. https://doi.org/10.32604/biocell.2021.013452
Goldman SM, Kamel F, Ross GW et al (2014) Peptidoglycan recognition protein genes and risk of Parkinson’s disease. Mov Disord 29:1171–1180. https://doi.org/10.1002/MDS.25895
Wasser CI, Mercieca EC, Kong G et al (2020) Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun 2. https://doi.org/10.1093/BRAINCOMMS/FCAA110
Kong G, Cao KAL, Judd LM et al (2020) Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiol Dis 135:104268. https://doi.org/10.1016/J.NBD.2018.09.001
Sun P, Su L, Zhu H et al (2021) Gut microbiota regulation and their implication in the development of neurodegenerative disease. Microorg 2281(9):2281. https://doi.org/10.3390/MICROORGANISMS9112281
Radulescu CI, Garcia-Miralles M, Sidik H et al (2020) Reprint of: Manipulation of microbiota reveals altered callosal myelination and white matter plasticity in a model of Huntington disease. Neurobiol Dis 135:104744. https://doi.org/10.1016/J.NBD.2020.104744
Kho ZY, Lal SK (2018) The human gut microbiome - a potential controller of wellness and disease. Front Microbiol 9:1835. https://doi.org/10.3389/FMICB.2018.01835/BIBTEX
Kim DS, Zhang T, Park S (2022) Protective effects of Forsythiae fructus and Cassiae semen water extract against memory deficits through the gut-microbiome-brain axis in an Alzheimer’s disease model. Pharm Biol 60:212. https://doi.org/10.1080/13880209.2022.2025860
Wang N, Feng B-N, Hu B et al (2022) Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct 13:2019–2032. https://doi.org/10.1039/D1FO02216D
Xu Z, Liu Z, Dong X et al (2018) Fecal microbiota transplantation from healthy donors reduced alcohol-induced anxiety and depression in an animal model of chronic alcohol exposure. Chin J Physiol 61:360–371. https://doi.org/10.4077/CJP.2018.BAH633
Leclercq S, Le Roy T, Furgiuele S et al (2020) Gut microbiota-induced changes in β-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep 33:108238. https://doi.org/10.1016/J.CELREP.2020.108238
Hu P, Wang D, Zhang Y et al (2020) Apoptosis-triggered decline in hippocampal microglia mediates adolescent intermittent alcohol exposure-induced depression-like behaviors in mice. Neuropharmacology 170:108054. https://doi.org/10.1016/J.NEUROPHARM.2020.108054
Wen XH, Ge C, Xing FG et al (2018) Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett 287:23–30. https://doi.org/10.1016/J.TOXLET.2018.01.021
Ferragud A, Velazquez-Sanchez C, Minnig MA et al (2020) Pituitary adenylate cyclase-activating polypeptide (PACAP) modulates dependence-induced alcohol drinking and anxiety-like behavior in male rats. Neuropsychopharmacol 463(46):509–518. https://doi.org/10.1038/s41386-020-00904-4
Mielenz D, Reichel M, Jia T et al (2018) EFhd2/Swiprosin-1 is a common genetic determinator for sensation-seeking/low anxiety and alcohol addiction. Mol Psychiatry 235(23):1303–1319. https://doi.org/10.1038/mp.2017.63
Frontera JL, Gonzalez Pini VM, Messore FL, Brusco A (2018) Exposure to cannabinoid agonist WIN 55,212-2 during early adolescence increases alcohol preference and anxiety in CD1 mice. Neuropharmacology 137:268–274. https://doi.org/10.1016/J.NEUROPHARM.2018.05.018
León BE, Kang S, Franca-Solomon G et al (2021) Alcohol-Induced neuroinflammatory response and mitochondrial dysfunction on aging and Alzheimer’s disease. Front Behav Neurosci 15. https://doi.org/10.3389/FNBEH.2021.778456
Hoffman JL, Faccidomo S, Kim M et al (2019) Alcohol drinking exacerbates neural and behavioral pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Int Rev Neurobiol 148:169–230. https://doi.org/10.1016/BS.IRN.2019.10.017
Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG (2019) Alcohol consumption and risk for Parkinson’s disease: a systematic review and meta-analysis. J Neurol 266:1821–1834. https://doi.org/10.1007/S00415-018-9032-3/FIGURES/5
Ghosh A, Giese KP (2015) Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol Brain 8:1–7. https://doi.org/10.1186/S13041-015-0166-2/FIGURES/1
Robison AJ (2014) Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci 37:653–662. https://doi.org/10.1016/J.TINS.2014.07.001
Ma H, Groth RD, Cohen SM et al (2014) γCaMKII Shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159:281–294. https://doi.org/10.1016/J.CELL.2014.09.019
Ortega-Martínez S (2015) A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci 8:46. https://doi.org/10.3389/FNMOL.2015.00046/BIBTEX
Cohen SM (2016) Signaling to the nucleus in excitatory and inhibitory neurons (Order No. 10139606). Available from Publicly Available Content Database. (1820070931). Retrieved from https://www.proquest.com/dissertations-theses/signaling-nucleus-excitatory-inhibitory-neurons/docview/1820070931/se-2
Zamarbide M, Mossa A, Muñoz-Llancao P et al (2019) Male-specific cAMP signaling in the hippocampus controls spatial memory deficits in a mouse model of autism and intellectual disability. Biol Psychiatry 85:760–768. https://doi.org/10.1016/J.BIOPSYCH.2018.12.013
Androschuk A, Al-Jabri B, Bolduc FV (2015) From learning to memory: what flies can tell us about intellectual disability treatment. Front Psychiatry 6:85. https://doi.org/10.3389/FPSYT.2015.00085/BIBTEX
Souza MA, Magni DV, Guerra GP et al (2012) Involvement of hippocampal CAMKII/CREB signaling in the spatial memory retention induced by creatine. Amino Acids 43:2491–2503. https://doi.org/10.1007/S00726-012-1329-4/TABLES/2
Zhang K, Liu R, Zhang J et al (2021) Electroacupuncture ameliorates depression-like behaviour in rats by enhancing synaptic plasticity via the GluN2B/CaMKII/ CREB signalling pathway. Evidence-Based Complement Altern Med 2021. https://doi.org/10.1155/2021/2146001
Misrani A, Tabassum S, Wang M et al (2020) Citalopram prevents sleep-deprivation-induced reduction in CaMKII-CREB-BDNF signaling in mouse prefrontal cortex. Brain Res Bull 155:11–18. https://doi.org/10.1016/J.BRAINRESBULL.2019.11.007
Islam R, Matsuzaki K, Sumiyoshi E et al (2019) Theobromine improves working memory by activating the CaMKII/CREB/BDNF pathway in rats. Nutr 888(11):888. https://doi.org/10.3390/NU11040888
Shen J, Yang L, Wei W (2021) Role of Fto on CaMKII/CREB signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav Brain Res 406:113227. https://doi.org/10.1016/J.BBR.2021.113227
Wu ZM, Ni GL, Shao AM, Cui R (2017) Genistein alleviates anxiety-like behaviors in post-traumatic stress disorder model through enhancing serotonergic transmission in the amygdala. Psychiatry Res 255:287–291. https://doi.org/10.1016/J.PSYCHRES.2017.05.051
Wang XL, Deng YX, Gao YM et al (2020) Activation of α7 nAChR by PNU-282987 improves synaptic and cognitive functions through restoring the expression of synaptic-associated proteins and the CaM-CaMKII-CREB signaling pathway. Aging (Albany NY) 12:543. https://doi.org/10.18632/AGING.102640
Xie W, Meng X, Zhai Y et al (2019) Antidepressant-like effects of the Guanxin Danshen formula via mediation of the CaMK II-CREB-BDNF signalling pathway in chronic unpredictable mild stress-induced depressive rats. Ann Transl Med 7:564–564. https://doi.org/10.21037/ATM.2019.09.39
Song X, Cui Z, He J et al (2021) κ-opioid receptor agonist, U50488H, inhibits pyroptosis through NLRP3 via the Ca2+/CaMKII/CREB signaling pathway and improves synaptic plasticity in APP/PS1 mice. Mol Med Rep 24:1–9. https://doi.org/10.3892/MMR.2021.12168/HTML
Martini F, Régis Leite M, Gonçalves Rosa S et al (2020) Strength exercise suppresses STZ-induced spatial memory impairment and modulates BDNF/ERK-CAMKII/CREB signalling pathway in the hippocampus of mice. Cell Biochem Funct 38:213–221. https://doi.org/10.1002/CBF.3470
Zhong J, Yu H, Huang C et al (2018) Inhibition of phosphodiesterase 4 by FCPR16 protects SH-SY5Y cells against MPP+-induced decline of mitochondrial membrane potential and oxidative stress. Redox Biol 16:47–58. https://doi.org/10.1016/J.REDOX.2018.02.008
Bilge SS, Günaydin C, Önger ME et al (2020) Neuroprotective action of agmatine in rotenone-induced model of Parkinson’s disease: role of BDNF/cREB and ERK pathway. Behav Brain Res 392:112692. https://doi.org/10.1016/J.BBR.2020.112692
Sun C, Wang Y, Mo M et al (2019) Minocycline protects against rotenone-induced neurotoxicity correlating with upregulation of Nurr1 in a Parkinson’s disease rat model. Biomed Res Int 2019. https://doi.org/10.1155/2019/6843265
Song Y, Zhao X, Wang D et al (2019) Inhibition of LPS-induced brain injury by NR2B antagonists through reducing assembly of NR2B–CaMKII–PSD95 signal module. Immunopharmacol Immunotoxicol 41:86–94. https://doi.org/10.1080/08923973.2018.1549566
Ning B, Zhang Q, Wang N et al (2019) β-Asarone regulates ER stress and autophagy via inhibition of the PERK/CHOP/Bcl-2/Beclin-1 pathway in 6-OHDA-induced Parkinsonian rats. Neurochem Res 44:1159–1166. https://doi.org/10.1007/S11064-019-02757-W/TABLES/1
Medina JH, Viola H (2018) ERK1/2: a key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci 11:361. https://doi.org/10.3389/FNMOL.2018.00361/BIBTEX
Lyons MR, West AE (2011) Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog Neurobiol 94:259. https://doi.org/10.1016/J.PNEUROBIO.2011.05.003
Melgarejo da Rosa M, Yuanxiang PA, Brambilla R et al (2016) Synaptic GluN2B/CaMKII-α signaling induces synapto-nuclear transport of ERK and Jacob. Front Mol Neurosci 9:66. https://doi.org/10.3389/FNMOL.2016.00066/BIBTEX
Ko MJ, Chiang T, Mukadam AA et al (2021) β-arrestin–dependent ERK signaling reduces anxiety-like and conditioned fear-related behaviors in mice. Sci Signal 14. https://doi.org/10.1126/SCISIGNAL.ABA0245
Sun S, Zhou J, Li Z et al (2022) Progranulin promotes hippocampal neurogenesis and alleviates anxiety-like behavior and cognitive impairment in adult mice subjected to cerebral ischemia. CNS Neurosci Ther. https://doi.org/10.1111/CNS.13810
Chuang HW, Wang TY, Huang CC, Wei IH (2022) Echinacoside exhibits antidepressant-like effects through AMPAR–Akt/ERK–mTOR pathway stimulation and BDNF expression in mice. Chinese Med (United Kingdom) 17:1–12. https://doi.org/10.1186/S13020-021-00549-5/FIGURES/6
Sun Z, Jia L, Shi D et al (2022) Deep brain stimulation improved depressive-like behaviors and hippocampal synapse deficits by activating the BDNF/mTOR signaling pathway. Behav Brain Res 419:113709. https://doi.org/10.1016/J.BBR.2021.113709
Ma QL, Harris-White ME, Ubeda OJ et al (2007) Evidence of Aβ- and transgene-dependent defects in ERK-CREB signaling in Alzheimer’s models. J Neurochem 103:1594–1607. https://doi.org/10.1111/J.1471-4159.2007.04869.X
Cao Q, Qin L, Huang F et al (2017) Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol Appl Pharmacol 319:80–90. https://doi.org/10.1016/J.TAAP.2017.01.019
Bohush A, Niewiadomska G, Filipek A (2018) Role of mitogen activated protein kinase signaling in Parkinson’s disease. Int J Mol Sci 2973(19):2973. https://doi.org/10.3390/IJMS19102973
Yusuf IO, Chen HM, Cheng PH et al (2019) Fibroblast growth factor 9 activates anti-oxidative functions of Nrf2 through ERK signalling in striatal cell models of Huntington’s disease. Free Radic Biol Med 130:256–266. https://doi.org/10.1016/J.FREERADBIOMED.2018.10.455
Rai SN, Dilnashin H, Birla H et al (2019) The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res 353(35):775–795. https://doi.org/10.1007/S12640-019-0003-Y
Gan X, Huang S, Wu L et al (2014) Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim Biophys Acta - Mol Basis Dis 1842:220–231. https://doi.org/10.1016/J.BBADIS.2013.11.009
Santini E, Heiman M, Greengard P et al (2009) Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal 2. https://doi.org/10.1126/SCISIGNAL.2000308/SUPPL_FILE/2_RA36_SM.PDF
Maher P, Dargusch R, Bodai L et al (2011) ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet 20:261–270. https://doi.org/10.1093/HMG/DDQ460
Beaver M, Bhatnagar A, Panikker P et al (2020) Disruption of Tip60 HAT mediated neural histone acetylation homeostasis is an early common event in neurodegenerative diseases. Sci Rep 10. https://doi.org/10.1038/s41598-020-75035-3
Esteves AR, Palma AM, Gomes R et al (2019) Acetylation as a major determinant to microtubule-dependent autophagy: relevance to Alzheimer’s and Parkinson disease pathology. Biochim Biophys Acta - Mol Basis Dis. https://doi.org/10.1016/j.bbadis.2018.11.014
Beaver M, Karisetty BC, Zhang H et al (2021) Chromatin and transcriptomic profiling uncover dysregulation of the Tip60 HAT/HDAC2 epigenomic landscape in the neurodegenerative brain. Epigenetics. https://doi.org/10.1080/15592294.2021.1959742
Leite JA, Ghirotto B, Targhetta VP et al (2022) Sirtuins as pharmacological targets in neurodegenerative and neuropsychiatric disorders. Br J Pharmacol 179:1496–1511
Manjula R, Anuja K, Alcain FJ (2021) SIRT1 and SIRT2 activity control in neurodegenerative diseases. Front Pharmacol 11
Zhang H, Karisetty BC, Bhatnagar A et al (2020) Tip60 protects against amyloid-β-induced transcriptomic alterations via different modes of action in early versus late stages of neurodegeneration. Mol Cell Neurosci 109. https://doi.org/10.1016/j.mcn.2020.103570
Takasu K, Niidome K, Hasegawa M, Ogawa K (2021) Histone deacetylase inhibitor improves the dysfunction of hippocampal gamma oscillations and fast spiking interneurons in Alzheimer’s disease model mice. Front Mol Neurosci 14. https://doi.org/10.3389/fnmol.2021.782206
Mazzocchi M, Goulding SR, Wyatt SL et al (2021) LMK235, a small molecule inhibitor of HDAC4/5, protects dopaminergic neurons against neurotoxin- and α-synuclein-induced degeneration in cellular models of Parkinson’s disease. Mol Cell Neurosci 115:103642. https://doi.org/10.1016/J.MCN.2021.103642
Mazzocchi M, Goulding SR, Morales-Prieto N et al (2022) Peripheral administration of the Class-IIa HDAC inhibitor MC1568 partially protects against nigrostriatal neurodegeneration in the striatal 6-OHDA rat model of Parkinson’s disease. Brain Behav Immun 102:151–160. https://doi.org/10.1016/j.bbi.2022.02.025
Burg T, Rossaert E, Moisse M et al (2021) Histone deacetylase inhibition regulates lipid homeostasis in a mouse model of amyotrophic lateral sclerosis. Int J Mol Sci 22. https://doi.org/10.3390/ijms222011224
Rossaert E, Pollari E, Jaspers T et al (2019) Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol Commun 7:107. https://doi.org/10.1186/s40478-019-0750-2
Pigna E, Simonazzi E, Sanna K et al (2019) Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine 40:717–732. https://doi.org/10.1016/j.ebiom.2019.01.038
Stoklund Dittlau K, Krasnow EN, Fumagalli L et al (2021) Human motor units in microfluidic devices are impaired by FUS mutations and improved by HDAC6 inhibition. Stem Cell Reports 16:2213–2227. https://doi.org/10.1016/j.stemcr.2021.03.029
Hecklau K, Mueller S, Koch SP et al (2021) The effects of selective inhibition of histone deacetylase 1 and 3 in Huntington’s disease Mice. Front Mol Neurosci 14. https://doi.org/10.3389/fnmol.2021.616886
Gilbert TM, Zürcher NR, Wu CJ et al (2019) PET neuroimaging reveals histone deacetylase dysregulation in schizophrenia. J Clin Invest 129. https://doi.org/10.1172/JCI123743
Ibi D, De La Fuente RM, Kezunovic N et al (2017) Antipsychotic-induced Hdac2 transcription via NF-κB leads to synaptic and cognitive side effects. Nat Neurosci 20:1247–1259. https://doi.org/10.1038/nn.4616
de la Fuente RM, Ibi D, Saunders JM et al (2018) HDAC2-dependent antipsychotic-like effects of chronic treatment with the HDAC inhibitor SAHA in mice. Neuroscience 388:102–117. https://doi.org/10.1016/j.neuroscience.2018.07.010
Tseng CEJ, Gilbert TM, Catanese MC et al (2020) In vivo human brain expression of histone deacetylases in bipolar disorder. Transl Psychiatry 10. https://doi.org/10.1038/s41398-020-00911-5
Iaconelli J, Xuan L, Karmacharya R (2019) HDAC6 modulates signaling pathways relevant to synaptic biology and neuronal differentiation in human stem cell-derived neurons. Int J Mol Sci 20
Qin L, Ma K, Yan Z (2021) Rescue of histone hypoacetylation and social deficits by ketogenic diet in a Shank3 mouse model of autism. Neuropsychopharmacology 47(6):1271–1279
Ma K, Qin L, Matas E et al (2018) Histone deacetylase inhibitor MS-275 restores social and synaptic function in a Shank3-deficient mouse model of autism. Neuropsychopharmacology 43:1779–1788
Qin L, Ma K, Wang ZJ et al (2018) Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat Neurosci 21:564–575. https://doi.org/10.1038/s41593-018-0110-8
Liu H, Tan M, Cheng B et al (2021) Valproic acid induces autism-like synaptic and behavioral deficits by disrupting histone acetylation of prefrontal cortex ALDH1A1 in rats. Front Neurosci 15. https://doi.org/10.3389/fnins.2021.641284
Basu T, O’Riordan KJ, Schoenike BA et al (2019) Histone deacetylase inhibitors restore normal hippocampal synaptic plasticity and seizure threshold in a mouse model of Tuberous Sclerosis Complex. Sci Rep 9. https://doi.org/10.1038/s41598-019-41744-7
Mishra R, Amanullah A, Upadhyay A et al (2020) Ubiquitin ligase LRSAM1 suppresses neurodegenerative diseases linked aberrant proteins induced cell death. Int J Biochem Cell Biol 120. https://doi.org/10.1016/j.biocel.2020.105697
Potjewyd FM, Axtman AD (2021) Exploration of aberrant E3 ligases implicated in Alzheimer’s disease and development of chemical tools to modulate their function. Front Cell Neurosci 15
Wang XL, Feng ST, Wang ZZ et al (2021) Parkin, an E3 ubiquitin ligase, plays an essential role in mitochondrial quality control in Parkinson’s disease. Cell Mol Neurobiol 41:1395–1411
Watabe K, Kato Y, Sakuma M et al (2020) Praja1 RING-finger E3 ubiquitin ligase suppresses neuronal cytoplasmic TDP-43 aggregate formation. Neuropathology 40:570–586. https://doi.org/10.1111/neup.12694
Jansen S, van der Werf IM, Innes AM et al (2019) De novo variants in FBXO11 cause a syndromic form of intellectual disability with behavioral problems and dysmorphisms. Eur J Hum Genet 27:738–746. https://doi.org/10.1038/s41431-018-0292-2
Rapanelli M, Tan T, Wang W et al (2021) Behavioral, circuitry, and molecular aberrations by region-specific deficiency of the high-risk autism gene Cul3. Mol Psychiatry 26:1491–1504. https://doi.org/10.1038/s41380-019-0498-x
Amar M, Pramod AB, Yu NK et al (2021) Autism-linked Cullin3 germline haploinsufficiency impacts cytoskeletal dynamics and cortical neurogenesis through RhoA signaling. Mol Psychiatry 26:3586–3613. https://doi.org/10.1038/s41380-021-01052-x
Kohlbrenner EA, Shaskan N, Pietersen CY et al (2018) Gene expression profile associated with postnatal development of pyramidal neurons in the human prefrontal cortex implicates ubiquitin ligase E3 in the pathophysiology of schizophrenia onset. J Psychiatr Res 102:110–117. https://doi.org/10.1016/j.jpsychires.2018.03.013
Strohmeyer R, Shelton J, Lougheed C, Breitkopf T (2014) CCAAT-enhancer binding protein-β expression and elevation in Alzheimer’s disease and microglial cell cultures. PLoS One 9. https://doi.org/10.1371/journal.pone.0086617
Ndoja A, Reja R, Lee SH et al (2020) Ubiquitin ligase COP1 Suppresses neuroinflammation by degrading c/EBPβ in microglia. Cell 182:1156–1169.e12
Chung E, Choi Y, Park J et al (2020) Intracellular delivery of Parkin rescues neurons from accumulation of damaged mitochondria and pathological α-synuclein. Sci Adv 6
Weskamp K, Barmada SJ (2018) TDP43 and RNA instability in amyotrophic lateral sclerosis. Brain Res 1693:67–74
Ma P, Li Y, Wang H, Mao B (2021) Haploinsufficiency of the TDP43 ubiquitin E3 ligase RNF220 leads to ALS-like motor neuron defects in the mouse. J Mol Cell Biol 13:374–382. https://doi.org/10.1093/jmcb/mjaa072
Lee YC, Huang WC, Lin JH et al (2018) Znf179 E3 ligase-mediated TDP-43 polyubiquitination is involved in TDP-43- ubiquitinated inclusions (UBI) (+)-related neurodegenerative pathology 11 Medical and Health Sciences. J Biomed Sci 25. https://doi.org/10.1186/s12929-018-0479-4
Koyuncu S, Saez I, Lee HJ et al (2018) The ubiquitin ligase UBR5 suppresses proteostasis collapse in pluripotent stem cells from Huntington’s disease patients. Nat Commun 9. https://doi.org/10.1038/s41467-018-05320-3
Yang G, Wan P, Xiang Q et al (2021) E3 ubiquitin ligase ASB17 promotes apoptosis by ubiquitylating and degrading BCLW and MCL1. Biology (Basel) 10. https://doi.org/10.3390/biology10030234
Farhang S, Sabaie H, Gharesouran J et al (2022) Expression analysis of ermin and listerin E3 ubiquitin protein ligase 1 genes in the periphery of patients with schizophrenia. J Mol Neurosci 72:246–254
Shiva S, Gharesouran J, Sabaie H et al (2021) Expression analysis of ermin and listerin E3 ubiquitin protein ligase 1 genes in autistic patients. Front Mol Neurosci 14. https://doi.org/10.3389/fnmol.2021.701977
Bi X, Cui K, Han C et al (2015) Association of NEDD4 gene polymorphisms with schizophrenia and its clinical characteristics in Chinese Han population. Chinese J Med Genet 32:385–390
Han C, Cui K, Bi X et al (2019) Association between polymorphism of the NEDD4 gene and cognitive dysfunction of schizophrenia patients in Chinese Han population. BMC Psychiatry 19. https://doi.org/10.1186/s12888-019-2386-y
Wang W, Qiao Y, Qu H et al (2020) The protective role of Neuregulin1-ErbB4 signaling in a chronic social defeat stress model. Neuroreport 678–685. https://doi.org/10.1097/WNR.0000000000001464
Xu J, Guo C, Liu Y et al (2020) Nedd4l downregulation of NRG1 in the mPFC induces depression-like behaviour in CSDS mice. Transl Psychiatry 10. https://doi.org/10.1038/s41398-020-00935-x
Kim S, Zhang S, Choi KH et al (2009) An E3 ubiquitin ligase, really interesting new gene (RING) finger 41, is a candidate gene for anxiety-like behavior and β-carboline-induced seizures. Biol Psychiatry 65:425–431. https://doi.org/10.1016/j.biopsych.2008.09.015
Lopez SJ, Segal DJ, LaSalle JM (2019) UBE3A: an E3 ubiquitin ligase with genome-wide impact in neurodevelopmental disease. Front Mol Neurosci 11
Yi JJ, Paranjape SR, Walker MP et al (2017) The autism-linked UBE3A T485A mutant E3 ubiquitin ligase activates the Wnt/β-catenin pathway by inhibiting the proteasome. J Biol Chem 292:12503–12515. https://doi.org/10.1074/jbc.M117.788448
Dong Z, Chen W, Chen C et al (2020) CUL3 deficiency causes social deficits and anxiety-like behaviors by impairing excitation-inhibition balance through the promotion of Cap-dependent translation. Neuron 105:475–490.e6. https://doi.org/10.1016/j.neuron.2019.10.035
Kaur G, Rathod SSS, Ghoneim MM et al (2022) DNA methylation: a promising approach in management of Alzheimer’s disease and other neurodegenerative disorders. Biology (Basel) 11
Bayer C, Pitschelatow G, Hannemann N et al (2020) DNA methyltransferase 1 (DNMT1) acts on neurodegeneration by modulating proteostasis-relevant intracellular processes. Int J Mol Sci 21:1–18. https://doi.org/10.3390/ijms21155420
Zsindely N, Siági F, Bodai L (2021) DNA methylation in huntington’s disease. Int J Mol Sci 22
Tenorio J, Alarcón P, Arias P et al (2020) Further delineation of neuropsychiatric findings in Tatton-Brown-Rahman syndrome due to disease-causing variants in DNMT3A: seven new patients. Eur J Hum Genet 28:469–479. https://doi.org/10.1038/s41431-019-0485-3
Wang H, Zhang B, Zeng B et al (2018) Association between catechol-O-methyltransferase genetic variation and functional connectivity in patients with first-episode schizophrenia. Schizophr Res 199:214–220. https://doi.org/10.1016/j.schres.2018.04.023
Zhang HQ, Wang JY, Li ZF et al (2021) DNA Methyltransferase 1 is dysregulated in Parkinson’s disease via mediation of miR-17. Mol Neurobiol 58:2620–2633. https://doi.org/10.1007/s12035-021-02298-w
Cali CP, Park DS, Lee EB (2019) Targeted DNA methylation of neurodegenerative disease genes via homology directed repair. Nucleic Acids Res 47:11609–11622. https://doi.org/10.1093/nar/gkz979
Pan Y, Daito T, Sasaki Y et al (2016) Inhibition of DNA methyltransferases blocks mutant huntingtin-induced neurotoxicity. Sci Rep 6. https://doi.org/10.1038/srep31022
Alex AM, Saradalekshmi KR, Shilen N et al (2019) Genetic association of DNMT variants can play a critical role in defining the methylation patterns in autism. IUBMB Life 71:901–907. https://doi.org/10.1002/iub.2021
Christian DL, Wu DY, Martin JR et al (2020) DNMT3A Haploinsufficiency results in behavioral deficits and global epigenomic dysregulation shared across neurodevelopmental disorders. Cell Rep 33. https://doi.org/10.1016/j.celrep.2020.108416
Cui HZ, Sun MZ, Wang RZ et al (2020) DNA methylation in the medial prefrontal cortex regulates alcohol-related behavior in rats. Yi Chuan = Hered 42:112–125. https://doi.org/10.16288/j.yczz.19-261
D’Addario C, Palazzo MC, Benatti B et al (2018) Regulation of gene transcription in bipolar disorders: Role of DNA methylation in the relationship between prodynorphin and brain derived neurotrophic factor. Prog Neuro-Psychopharmacology Biol Psychiatry 82:314–321. https://doi.org/10.1016/j.pnpbp.2017.08.011
Zhang L, Pang B, Zhang W et al (2018) Association between schizophrenia and DNA demethylase activity in human peripheral blood mononuclear cells. Clin Lab 64:1031–1035. https://doi.org/10.7754/Clin.Lab.2018.180127
Saxena S, Choudhury S, Maroju PA et al (2021) Dysregulation of schizophrenia-Associated genes and genome-wide hypomethylation in neurons overexpressing DNMT1. Epigenomics 13:1539–1555. https://doi.org/10.2217/epi-2021-0133
Saxena S, Maroju PA, Choudhury S et al (2020) Analysis of transcript levels of a few schizophrenia candidate genes in neurons from a transgenic mouse embryonic stem cell model overexpressing DNMT1. Gene 757. https://doi.org/10.1016/j.gene.2020.144934
Srancikova A, Reichova A, Bacova Z, Bakos J (2021) Gene expression levels of DNA methyltransferase enzymes in Shank3-deficient mouse model of autism during early development. Endocr Regul 55:234–237. https://doi.org/10.2478/enr-2021-0025
Nagarajan RP, Hogart AR, Gwye Y et al (2006) Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics 1:172–182. https://doi.org/10.4161/epi.1.4.3514
Lu Z, Liu Z, Mao W et al (2020) Locus-specific DNA methylation of Mecp2 promoter leads to autism-like phenotypes in mice. Cell Death Dis 11. https://doi.org/10.1038/s41419-020-2290-x
Gao Y, Duque-Wilckens N, Aljazi MB et al (2021) Loss of histone methyltransferase ASH1L in the developing mouse brain causes autistic-like behaviors. Commun Biol 4. https://doi.org/10.1038/s42003-021-02282-z
Pawar S, Bellver-Sanchis A, Griñán-Ferré C (2021) RNA-seq and miRNA-seq data from pharmacological inhibition of the G9a/GLP histone methyltransferase complex with UNC0642 in SAMP8 mice. Data Br 36. https://doi.org/10.1016/j.dib.2021.107114
Griñán-Ferré C, Marsal-García L, Bellver-Sanchis A et al (2019) Pharmacological inhibition of G9a/GLP restores cognition and reduces oxidative stress, neuroinflammation and β-amyloid plaques in an early-onset Alzheimer’s disease mouse model. Aging (Albany NY) 11:11591–11608. https://doi.org/10.18632/aging.102558
Wang DY, Kosowan J, Samsom J et al (2018) Inhibition of the G9a/GLP histone methyltransferase complex modulates anxiety-related behavior in mice. Acta Pharmacol Sin 39:866–874. https://doi.org/10.1038/aps.2017.190
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335. https://doi.org/10.1021/NP200906S
Mok SWF, Wong VKW, Lo HH et al (2020) Natural products-based polypharmacological modulation of the peripheral immune system for the treatment of neuropsychiatric disorders. Pharmacol Ther 208. https://doi.org/10.1016/J.PHARMTHERA.2020.107480
Sarris J, Marx W, Ashton MM et al (2021) Plant-based medicines (phytoceuticals) in the treatment of psychiatric disorders: a meta-review of meta-analyses of randomized controlled trials: Les médicaments à base de plantes (phytoceutiques) dans le traitement des troubles psychiatriques: une méta-r. Can J Psychiatry 66:849–862. https://doi.org/10.1177/0706743720979917
Kumar GP, Khanum F (2012) Neuroprotective potential of phytochemicals. Pharmacogn Rev 6:81. https://doi.org/10.4103/0973-7847.99898
Aryal B, Raut BK, Bhattarai S et al (2022) Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases. Evidence-Based Complement Altern Med 2022:1–18. https://doi.org/10.1155/2022/7299778
Küpeli Akkol E, Tatlı Çankaya I, Şeker Karatoprak G et al (2021) Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front Pharmacol 12. https://doi.org/10.3389/FPHAR.2021.669638/FULL
Agarwal B, Pal P, Parihar L et al (2022) Evaluation of protective effect of vinpocetine in reserpine-induced depression in Wistar rats. J Posit Sch Psychol 6:2471–2481
Sheref AA, Naguib YM, Abou-Elnour ES et al (2022) Neuroprotective effect of piracetam and vincamine in a rat model of haloperidol-induced Parkinson’s disease. Bull Egypt Soc Physiol Sci 42:11–26. https://doi.org/10.21608/BESPS.2021.71203.1099
Leri M, Scuto M, Ontario ML et al (2020) Healthy effects of plant polyphenols: molecular mechanisms. Int J Mol Sci 21:1250. https://doi.org/10.3390/IJMS21041250
Casamenti F, Stefani M (2017) Olive polyphenols: new promising agents to combat aging-associated neurodegeneration. Expert Rev Neurother 17:345–358. https://doi.org/10.1080/14737175.2017.1245617
Carito V, Ceccanti M, Tarani L et al (2016) Neurotrophins’ modulation by olive polyphenols. Curr Med Chem 23:3189–3197. https://doi.org/10.2174/0929867323666160627104022
Donoso F, Egerton S, Bastiaanssen TFS et al (2020) Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 116:104673. https://doi.org/10.1016/J.PSYNEUEN.2020.104673
Caracci F, Harary J, Simkovic S, Pasinetti GM (2019) Grape-derived polyphenols ameliorate stress-induced depression by regulating synaptic plasticity. J Agric Food Chem. https://doi.org/10.1021/ACS.JAFC.9B01970/ASSET/IMAGES/MEDIUM/JF9B01970_0003.GIF
Yuan Y, Zhen L, Li Z et al (2020) trans-Resveratrol ameliorates anxiety-like behaviors and neuropathic pain in mouse model of post-traumatic stress disorder. J Psychopharmacol 34:726–736. https://doi.org/10.1177/0269881120914221
Huang N, Zhang Y, Chen M et al (2019) Resveratrol delays 6-hydroxydopamine-induced apoptosis by activating the PI3K/Akt signaling pathway. Exp Gerontol 124:110653. https://doi.org/10.1016/J.EXGER.2019.110653
Espinosa J, Rocha A, Nunes F et al (2013) Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A 2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J Alzheimer’s Dis 34:509–518. https://doi.org/10.3233/JAD-111982
Basu Mallik S, Mudgal J, Hall S et al (2021) Remedial effects of caffeine against depressive-like behaviour in mice by modulation of neuroinflammation and BDNF. Nutr Neurosci 25:1836–1844. https://doi.org/10.1080/1028415X.2021.1906393
Duarte T, Fontana BD, Müller TE et al (2019) Nicotine prevents anxiety-like behavioral responses in zebrafish. Prog Neuro-Psychopharmacol Biol Psychiatry 94:109655. https://doi.org/10.1016/J.PNPBP.2019.109655
Carvajal-Oliveros A, Domínguez-Baleón C, Zárate RV et al (2021) Nicotine suppresses Parkinson’s disease like phenotypes induced by Synphilin-1 overexpression in Drosophila melanogaster by increasing tyrosine hydroxylase and dopamine levels. Sci Rep 111(11):1–13. https://doi.org/10.1038/s41598-021-88910-4
Sharma N, Soni R, Sharma M et al (2022) Chlorogenic acid: a polyphenol from coffee rendered neuroprotection against rotenone-induced Parkinson’s disease by GLP-1 secretion. Mol Neurobiol 59:6834–6856. https://doi.org/10.1007/S12035-022-03005-Z/FIGURES/9
Gao L, Li X, Meng S et al (2020) Chlorogenic acid alleviates Aβ25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des Devel Ther 14:1705–1716. https://doi.org/10.2147/DDDT.S235969
Chen X-D, Tang J-J, Feng S et al (2021) Chlorogenic acid improves PTSD-like symptoms and associated mechanisms. Curr Neuropharmacol 19:2180. https://doi.org/10.2174/1570159X19666210111155110
Ayaz M, Sadiq A, Junaid M et al (2019) Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front Aging Neurosci 11. https://doi.org/10.3389/FNAGI.2019.00155
Donato F, de Gomes MG, Goes ATR et al (2014) Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull 104:19–26. https://doi.org/10.1016/J.BRAINRESBULL.2014.03.004
Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR et al (2015) The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 93:134–145. https://doi.org/10.1016/J.NEUROPHARM.2015.01.027
Kim JK, Choi SJ, Cho HY et al (2014) Protective effects of kaempferol (3,4′,5,7-tetrahydroxyflavone) against amyloid beta peptide (Aβ)-induced neurotoxicity in ICR mice. OUP 74:397–401. https://doi.org/10.1271/BBB.90585
Merzoug S, Toumi ML, Tahraoui A (2014) Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedebergs Arch Pharmacol 387:921–933. https://doi.org/10.1007/S00210-014-1008-Y/TABLES/1
Gao W, Wang W, Peng Y, Deng Z (2019) Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab Brain Dis 34:485–494. https://doi.org/10.1007/S11011-019-0389-5/FIGURES/8
Sur B, Lee B (2022) Luteolin reduces fear, anxiety, and depression in rats with post-traumatic stress disorder. Animal Cells Syst (Seoul) 26:174. https://doi.org/10.1080/19768354.2022.2104925
Ahmad S, Jo MH, Ikram M et al (2021) Deciphering the potential neuroprotective effects of luteolin against Aβ1–42-induced Alzheimer’s disease. Int J Mol Sci 9583(22):9583. https://doi.org/10.3390/IJMS22179583
Li R, Wang X, Qin T et al (2016) Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res 296:318–325. https://doi.org/10.1016/J.BBR.2015.09.031
Zhao L, Wang JL, Liu R et al (2013) Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Mol 9949-9965(18):9949–9965. https://doi.org/10.3390/MOLECULES18089949
Pervin M, Unno K, Ohishi T et al (2018) Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 23. https://doi.org/10.3390/MOLECULES23061297
Maher P (2019) The potential of flavonoids for the treatment of neurodegenerative diseases. Int J Mol Sci 20. https://doi.org/10.3390/IJMS20123056
Ahmad MH, Fatima M, Ali M et al (2021) Naringenin alleviates paraquat-induced dopaminergic neuronal loss in SH-SY5Y cells and a rat model of Parkinson’s disease. Neuropharmacology 201:108831. https://doi.org/10.1016/J.NEUROPHARM.2021.108831
Tayyab M, Farheen S, Mariyath MPM et al (2019) Antidepressant and neuroprotective effects of naringenin via Sonic Hedgehog-GLI1 cell signaling pathway in a rat model of chronic unpredictable mild stress. NeuroMolecular Med 21:250–261. https://doi.org/10.1007/S12017-019-08538-6/FIGURES/6
Pannu A, Sharma PC, Thakur VK, Goyal RK (2021) Emerging role of flavonoids as the treatment of depression. Biomolecules 11. https://doi.org/10.3390/BIOM11121825
Manayi A, Nabavi SM, Daglia M, Jafari S (2016) Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol Rep 68:671–679. https://doi.org/10.1016/J.PHAREP.2016.03.014
Hu G, Gong X, Wang L et al (2017) Triptolide Promotes the clearance of α-synuclein by enhancing autophagy in neuronal cells. Mol Neurobiol 54:2361–2372. https://doi.org/10.1007/S12035-016-9808-3
Nazari L, Komaki S, Salehi I et al (2022) Investigation of the protective effects of lutein on memory and learning using behavioral methods in a male rat model of Alzheimer’s disease. J Funct Foods 99:105319. https://doi.org/10.1016/J.JFF.2022.105319
Zeni ALB, Camargo A, Dalmagro AP (2019) Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol Biochem Behav 179:63–72. https://doi.org/10.1016/J.PBB.2019.02.004
Zhang F, Fu Y, Zhou X et al (2016) Depression-like behaviors and heme oxygenase-1 are regulated by lycopene in lipopolysaccharide-induced neuroinflammation. J Neuroimmunol 298:1–8. https://doi.org/10.1016/J.JNEUROIM.2016.06.001
Kaur H, Chauhan S, Sandhir R (2011) Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem Res 36:1435–1443. https://doi.org/10.1007/S11064-011-0469-3/FIGURES/2
Selmin OI, Romagnolo APG, Romagnolo DF (2016) Mediterranean diet and neurodegenerative diseases. Mediterr Diet 153–164. https://doi.org/10.1007/978-3-319-27969-5_12
Armeli F, Bonucci A, Maggi E et al (2021) Mediterranean diet and neurodegenerative diseases: the neglected role of nutrition in the modulation of the endocannabinoid system. Biomol 11:790. https://doi.org/10.3390/BIOM11060790
Joseph J, Depp C, Shih PAB et al (2017) Modified Mediterranean diet for enrichment of short chain fatty acids: potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front Neurosci 11:155. https://doi.org/10.3389/FNINS.2017.00155/BIBTEX
Bayes J, Schloss J, Sibbritt D (2020) Effects of polyphenols in a Mediterranean diet on symptoms of depression: a systematic literature review. Adv Nutr 11:602–615. https://doi.org/10.1093/ADVANCES/NMZ117
Shafiei F, Salari-Moghaddam A, Larijani B et al (2019) Adherence to the Mediterranean diet and risk of depression: a systematic review and updated meta-analysis of observational studies. Nutr Rev 77:230–239. https://doi.org/10.1093/NUTRIT/NUY070
de la Rubia Ortí JE, García-Pardo MP, Drehmer E et al (2018) Improvement of main cognitive functions in patients with Alzheimer’s disease after treatment with coconut oil enriched Mediterranean diet: a pilot study. J Alzheimer’s Dis 65:577–587. https://doi.org/10.3233/JAD-180184
Paknahad Z, Sheklabadi E, Derakhshan Y et al (2020) The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: a randomized clinical controlled trial. Complement Ther Med 50:102366. https://doi.org/10.1016/J.CTIM.2020.102366
Visioli F, Rodríguez-Pérez M, Gómez-Torres Ó et al (2020) Hydroxytyrosol improves mitochondrial energetics of a cellular model of Alzheimer’s disease. https://doi.org/10.1080/1028415X.2020.1829344
Solch RJ, Aigbogun JO, Voyiadjis AG et al (2022) Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: a systematic review. J Neurol Sci 434:120166. https://doi.org/10.1016/J.JNS.2022.120166
Paknahad Z, Sheklabadi E, Moravejolahkami AR et al (2020) The effects of Mediterranean diet on severity of disease and serum total antioxidant capacity (TAC) in patients with Parkinson’s disease: a single center, randomized controlled trial. Nutr Neurosci 25:313–320. https://doi.org/10.1080/1028415X.2020.1751509
Malik J, Kaur S, Karan M, Choudhary S (2020) Neuroprotective effect of standardized extracts of three Lactuca sativa Linn. varieties against 3-NP induced Huntington’s disease like symptoms in rats. https://doi.org/10.1080/1028415X.2020.1841500
Knight A, Bryan J, Murphy K (2016) The Mediterranean diet and age-related cognitive functioning: a systematic review of study findings and neuropsychological assessment methodology. Nutr Neurosci 20:449–468. https://doi.org/10.1080/1028415X.2016.1183341
Abdolahi M, Karimi E, Sarraf P et al (2021) The omega-3 and nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: a randomized clinical trial. BMC Res Notes 14:1–7. https://doi.org/10.1186/S13104-021-05700-X/TABLES/3
de Andrade WK, de Sá Barreto da Cunha M, Miranda SS et al (2021) Non-specific effect of omega-3 fatty acid supplementation on autistic spectrum disorder: systematic review and meta-analysis. https://doi.org/10.1080/1028415X.2021.1913950
Bae JH, Kim G (2018) Systematic review and meta-analysis of omega-3-fatty acids in elderly patients with depression. Nutr Res 50:1–9. https://doi.org/10.1016/J.NUTRES.2017.10.013
Hsu MC, Tung CY, Chen HE (2018) Omega-3 polyunsaturated fatty acid supplementation in prevention and treatment of maternal depression: Putative mechanism and recommendation. J Affect Disord 238:47–61. https://doi.org/10.1016/J.JAD.2018.05.018
Pérez MÁ, Peñaloza-Sancho V, Ahumada J et al (2017) n-3 polyunsaturated fatty acid supplementation restored impaired memory and GABAergic synaptic efficacy in the hippocampus of stressed rats. Nutr Neurosci 21:556–569. https://doi.org/10.1080/1028415X.2017.1323609
Sharifi-Rad M, Lankatillake C, Dias DA et al (2020) Impact of natural compounds on neurodegenerative disorders: from preclinical to pharmacotherapeutics. J Clin Med 9:1061. https://doi.org/10.3390/jcm9041061
Lin J, Zhang X, Li C et al (2020) Evodiamine via targeting nNOS and AMPA receptor GluA1 inhibits nitroglycerin-induced migraine-like response. J Ethnopharmacol 254:112727. https://doi.org/10.1016/J.JEP.2020.112727
Sun L, Zhang W, Ye R et al (2021) Catalpol enhanced physical exercise-mediated brain functional improvement in post-traumatic stress disorder model via promoting adult hippocampal neurogenesis. Aging (Albany NY) 13:18689. https://doi.org/10.18632/AGING.203313
Alzoubi KH, Al Hilo AS, Al-Balas QA et al (2019) Withania somnifera root powder protects againist post-traumatic stress disorder-induced memory impairment. Mol Biol Rep 46:4709–4715. https://doi.org/10.1007/S11033-019-04915-3/FIGURES/4
Zhu J, Park S, Jeong KH, Kim WJ (2020) Withanolide-A treatment exerts a neuroprotective effect via inhibiting neuroinflammation in the hippocampus after pilocarpine-induced status epilepticus. Epilepsy Res 165. https://doi.org/10.1016/J.EPLEPSYRES.2020.106394
Zhu H, Wang G, Bai Y et al (2022) Natural bear bile powder suppresses neuroinflammation in lipopolysaccharide-treated mice via regulating TGR5/AKT/NF-κB signaling pathway. J Ethnopharmacol 289:115063. https://doi.org/10.1016/J.JEP.2022.115063
Soares-Silva B, Beserra-Filho JIA, Morera PMA et al (2022) The bee venom active compound melittin protects against bicuculline-induced seizures and hippocampal astrocyte activation in rats. Neuropeptides 91:102209. https://doi.org/10.1016/J.NPEP.2021.102209
Alvi AM, Al Kury LT, Alattar A et al (2021) Carveol attenuates seizure severity and neuroinflammation in pentylenetetrazole-kindled epileptic rats by regulating the Nrf2 signaling pathway. Oxid Med Cell Longev 2021. https://doi.org/10.1155/2021/9966663
Qusa MH, Abdelwahed KS, Meyer SA, El Sayed KA (2020) Olive oil lignan (+)-acetoxypinoresinol peripheral motor and neuronal protection against the tremorgenic mycotoxin penitrem A toxicity via STAT1 pathway. ACS Chem Neurosci 11:3575–3589. https://doi.org/10.1021/ACSCHEMNEURO.0C00458
Xi Y, Liu M, Xu S et al (2019) Inhibition of SERPINA3N-dependent neuroinflammation is essential for melatonin to ameliorate trimethyltin chloride–induced neurotoxicity. J Pineal Res 67:e12596. https://doi.org/10.1111/JPI.12596
Lee SH, Choi BY, Kho AR et al (2018) Inhibition of NADPH oxidase activation by apocynin rescues seizure-induced reduction of adult hippocampal neurogenesis. Int J Mol Sci 19. https://doi.org/10.3390/IJMS19103087
Rodríguez-Muñoz M, Onetti Y, Cortés-Montero E et al (2018) Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol Brain 11. https://doi.org/10.1186/S13041-018-0395-2
Madadzadeh M, Abbasnejad M, Mollashahi M et al (2021) Phytohormone abscisic acid boosts pentobarbital-induced sleep through activation of GABA-A, PPARβ and PPARγ receptor signaling. Arq Neuropsiquiatr 79:216–221. https://doi.org/10.1590/0004-282X-ANP-2019-0393
Oishi K, Okauchi H, Yamamoto S, Higo-Yamamoto S (2020) Dietary natural cocoa ameliorates disrupted circadian rhythms in locomotor activity and sleep-wake cycles in mice with chronic sleep disorders caused by psychophysiological stress. Nutrition 75–76:110751. https://doi.org/10.1016/J.NUT.2020.110751
Petrella C, Carito V, Carere C et al (2020) Oxidative stress inhibition by resveratrol in alcohol-dependent mice. Nutrition 79–80. https://doi.org/10.1016/J.NUT.2020.110783
Ben-Azu B, Nwoke EE, Aderibigbe AO et al (2019) Possible neuroprotective mechanisms of action involved in the neurobehavioral property of naringin in mice. Biomed Pharmacother 109:536–546. https://doi.org/10.1016/J.BIOPHA.2018.10.055
Joseph A, Thuy TTT, Thanh LT, Okada M (2018) Antidepressive and anxiolytic effects of ostruthin, a TREK-1 channel activator. PLoS One 13. https://doi.org/10.1371/JOURNAL.PONE.0201092
Zhang L, Liu C, Yuan M (2020) Eriodictyol produces antidepressant-like effects and ameliorates cognitive impairments induced by chronic stress. Neuroreport 1111–1120. https://doi.org/10.1097/WNR.0000000000001525
Zhang B, Li Y, Liu M et al (2020) Antidepressant-like mechanism of honokiol in a rodent model of corticosterone-induced depression. J Integr Neurosci 19:459–467. https://doi.org/10.31083/J.JIN.2020.03.172/1757-448X-19-3-459/FIG8.JPG
Yuan HL, Zhao YL, Ding CF et al (2020) Anti-inflammatory and antinociceptive effects of Curcuma kwangsiensis and its bioactive terpenoids in vivo and in vitro. J Ethnopharmacol 259:112935. https://doi.org/10.1016/J.JEP.2020.112935
Luduvico KP, Spohr L, Soares MSP et al (2020) Antidepressant effect and modulation of the redox system mediated by tannic acid on lipopolysaccharide-induced depressive and inflammatory changes in mice. Neurochem Res 45:2032–2043. https://doi.org/10.1007/S11064-020-03064-5/FIGURES/7
Fang A, Li Y, Wu X et al (2020) Baicalin attenuates inflammatory pain associated depressive symptoms via Akt-mediated adult hippocampal neurogenesis. Metab Brain Dis 35:1085–1093. https://doi.org/10.1007/S11011-020-00599-Y/FIGURES/4
Ma L, Hu P, Zhang J et al Purpurin exerted antidepressant-like effects on behavior and stress axis reactivity: evidence of serotonergic engagement. https://doi.org/10.1007/s00213-019-05422-w
Júnior ALG, Tchekalarova JD, da Conceição MK et al (2019) Antidepressant-like effect of anacardic acid in mice via the L-arginine–nitric oxide–serotonergic system. Phyther Res 33:2126–2138. https://doi.org/10.1002/PTR.6407
Fu S, Wang J, Hao C et al Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3 inflammasome signal pathway in mice. https://doi.org/10.1007/s00213-019-05210-6
Khan K, Najmi AK, Akhtar M (2019) A natural phenolic compound quercetin showed the usefulness by targeting inflammatory, oxidative stress markers and augment 5-HT levels in one of the animal models of depression in mice. Drug Res (Stuttg) 69:392–400. https://doi.org/10.1055/A-0748-5518
Nazir S, Farooq RK et al Therapeutic effect of thymoquinone on behavioural response to UCMS and neuroinflammation in hippocampus and amygdala in BALB/c mice model. Psychopharmacology (Berl) 1:3. https://doi.org/10.1007/s00213-021-06038-9
Vega Custódio S, Spohr L, Pontes Bona N et al (2021) Effect of blueberry (Vaccinium virgatum) extract on depressive-like behavior and metabolic serum alterations in lipopolysaccharide-challenged mice. J Food Biochem 45. https://doi.org/10.1111/JFBC.13920
Wei X, Ma Y, Li F et al (2021) Acute diallyl disulfide administration prevents and reveres lipopolysaccharide-induced depression-like behaviors in mice via regulating neuroinflammation and oxido-nitrosative stress. Inflammation 44:1381–1395. https://doi.org/10.1007/S10753-021-01423-0/FIGURES/7
Zhang T, Yang C, Chu J et al (2021) Emodin prevented depression in chronic unpredicted mild stress-exposed rats by targeting miR-139-5p/5-lipoxygenase. Front cell Dev Biol 9. https://doi.org/10.3389/FCELL.2021.696619
Tian DD, Wang M, Liu A et al (2021) Antidepressant Effect of paeoniflorin is through inhibiting pyroptosis CASP-11/GSDMD pathway. Mol Neurobiol 58:761–776. https://doi.org/10.1007/S12035-020-02144-5/FIGURES/11
Li Y, Song W, Tong Y et al (2021) Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J Neuroinflammation 18. https://doi.org/10.1186/S12974-020-02040-8
Norins LC (2021) Repurposing licensed drugs for use against Alzheimer’s disease. J Alzheimer’s Dis 81:921. https://doi.org/10.3233/JAD-210080
Fletcher EJR, Kaminski T, Williams G, Duty S (2021) Drug repurposing strategies of relevance for Parkinson’s disease. Pharmacol Res Perspect 9:e00841. https://doi.org/10.1002/PRP2.841
Ebada ME (2017) Drug repurposing may generate novel approaches to treating depression. J Pharm Pharmacol 69:1428–1436. https://doi.org/10.1111/JPHP.12815
Yang YS, Marder SR, Green MF (2017) Repurposing drugs for cognition in schizophrenia. Clin Pharmacol Ther 101:191–193. https://doi.org/10.1002/CPT.529
Ramsay RR, Majekova M, Medina M, Valoti M (2016) Key targets for multi-target ligands designed to combat neurodegeneration. Front Neurosci 10:375. https://doi.org/10.3389/FNINS.2016.00375/BIBTEX
Ramsay RR, Popovic-Nikolic MR, Nikolic K et al (2018) A perspective on multi-target drug discovery and design for complex diseases. Clin Transl Med 7:1–14. https://doi.org/10.1186/S40169-017-0181-2/FIGURES/4
Marzo A, Dal Bo L, Monti NC et al (2004) Pharmacokinetics and pharmacodynamics of safinamide, a neuroprotectant with antiparkinsonian and anticonvulsant activity. Pharmacol Res 50:77–85. https://doi.org/10.1016/J.PHRS.2003.12.004
Jaiteh M, Zeifman A, Saarinen M et al (2018) Docking screens for dual inhibitors of disparate drug targets for Parkinson’s disease. J Med Chem 61:5269–5278. https://doi.org/10.1021/ACS.JMEDCHEM.8B00204
Mesiti F, Chavarria D, Gaspar A et al (2019) The chemistry toolbox of multitarget-directed ligands for Alzheimer’s disease. Eur J Med Chem 181:111572. https://doi.org/10.1016/J.EJMECH.2019.111572
Jankowska A, Wesolowska A, Pawlowski M, Chlon-Rzepa G (2018) Multi-target-directed ligands affecting serotonergic neurotransmission for Alzheimer’s disease therapy: advances in chemical and biological research. Curr Med Chem 25:2045–2067. https://doi.org/10.2174/0929867324666170529122802
Hubsher G, Haider M, Okun MS (2012) Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology 78:1096–1099. https://doi.org/10.1212/WNL.0B013E31824E8F0D
Meythaler JM, Brunner RC, Johnson A, Novack TA (2002) Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: a pilot double-blind randomized trial. J Head Trauma Rehabil 17:300–313. https://doi.org/10.1097/00001199-200208000-00004
McLaren KD, Marangell LB (2004) Special considerations in the treatment of patients with bipolar disorder and medical co-morbidities. Ann Gen Hosp Psychiatry 3. https://doi.org/10.1186/1475-2832-3-7
Tousi B (2015) The emerging role of bexarotene in the treatment of Alzheimer’s disease: current evidence. Neuropsychiatr Dis Treat 11:311. https://doi.org/10.2147/NDT.S61309
Lerner V, Miodownik C, Gibel A et al (2013) The retinoid X receptor agonist bexarotene relieves positive symptoms of schizophrenia: a 6-week, randomized, double-blind, placebo-controlled multicenter trial. J Clin Psychiatry 74:1224–1232. https://doi.org/10.4088/JCP.12M08160
De La Garza R, Asnis GM (2003) The non-steroidal anti-inflammatory drug diclofenac sodium attenuates IFN-alpha induced alterations to monoamine turnover in prefrontal cortex and hippocampus. Brain Res 977:70–79. https://doi.org/10.1016/S0006-8993(03)02757-4
Stuve O, Weideman RA, McMahan DM et al (2020) Diclofenac reduces the risk of Alzheimer’s disease: a pilot analysis of NSAIDs in two US veteran populations. Ther Adv Neurol Disord 13:1756286420935676. https://doi.org/10.1177/1756286420935676
Carboni E, Carta AR, Carboni E, Novelli A (2021) Repurposing ketamine in depression and related disorders: can this enigmatic drug achieve success? Front Neurosci 15:469. https://doi.org/10.3389/FNINS.2021.657714/BIBTEX
Wang R, Zhang Z, Kumar M et al (2019) Neuroprotective potential of ketamine prevents developing brain structure impairment and alteration of neurocognitive function induced via isoflurane through the PI3K/AKT/GSK-3β pathway. Drug Des Devel Ther 13:501. https://doi.org/10.2147/DDDT.S188636
Liao W, Xu J, Li B et al (2022) Deciphering the roles of metformin in Alzheimer’s disease: a snapshot. Front Pharmacol 12:4123. https://doi.org/10.3389/FPHAR.2021.728315/BIBTEX
Hervás D, Fornés-Ferrer V, Gómez-Escribano AP et al (2017) Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS One 12:e0179283. https://doi.org/10.1371/JOURNAL.PONE.0179283
Wang X, Luo C, Mao XY et al (2019) Metformin reverses the schizophrenia-like behaviors induced by MK-801 in rats. Brain Res 1719:30–39. https://doi.org/10.1016/J.BRAINRES.2019.05.023
Belanoff JK, Jurik J, Schatzberg LD et al (2002) Slowing the progression of cognitive decline in Alzheimer’s disease using mifepristone. J Mol Neurosci 19:201–206. https://doi.org/10.1007/S12031-002-0033-3
Gallagher P, Watson S, Elizabeth Dye C et al (2008) Persistent effects of mifepristone (RU-486) on cortisol levels in bipolar disorder and schizophrenia. J Psychiatr Res 42:1037–1041. https://doi.org/10.1016/J.JPSYCHIRES.2007.12.005
Cankaya S, Cankaya B, Kilic U et al (2019) The therapeutic role of minocycline in Parkinson’s disease. Drugs Context 8. https://doi.org/10.7573/DIC.212553
Joel Yager M (2020) Minocycline as an adjunctive treatment for depression. NEJM J Watch 2020. https://doi.org/10.1056/NEJM-JW.NA52744
La Barbera L, Vedele F, Nobili A et al (2021) Nilotinib restores memory function by preventing dopaminergic neuron degeneration in a mouse model of Alzheimer’s Disease. Prog Neurobiol 202. https://doi.org/10.1016/J.PNEUROBIO.2021.102031
Krzystyniak A, Wesierska M, Petrazzo G et al (2022) Combination of dasatinib and quercetin improves cognitive abilities in aged male Wistar rats, alleviates inflammation and changes hippocampal synaptic plasticity and histone H3 methylation profile. Aging (Albany NY) 14:572–595. https://doi.org/10.18632/AGING.203835
Gonzales MM, Garbarino VR, Marques Zilli E et al (2022) Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): a pilot clinical trial. J Prev Alzheimer’s Dis 9:22–29. https://doi.org/10.14283/JPAD.2021.62
Galimberti D, Scarpini E (2016) Pioglitazone for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs 26:97–101. https://doi.org/10.1080/13543784.2017.1265504
Iranpour N, Zandifar A, Farokhnia M et al (2016) The effects of pioglitazone adjuvant therapy on negative symptoms of patients with chronic schizophrenia: a double-blind and placebo-controlled trial. Hum Psychopharmacol Clin Exp 31:103–112. https://doi.org/10.1002/HUP.2517
Sahli ZT, Tarazi FI (2018) Pimavanserin: novel pharmacotherapy for Parkinson’s disease psychosis. Expert Opin Drug Discov 13:103–110. https://doi.org/10.1080/17460441.2018.1394838
Pastoor D, Gobburu J (2014) Clinical pharmacology review of escitalopram for the treatment of depression. Expert Opin Drug Metab Toxicol 10:121–128. https://doi.org/10.1517/17425255.2014.863873
Wang YJ, Gong WG, Ren QG, Zhang ZJ (2020) Escitalopram alleviates Alzheimer’s disease-type tau pathologies in the aged P301L tau transgenic mice. J Alzheimers Dis 77:807–819. https://doi.org/10.3233/JAD-200401
Rong X, Jiang L, Qu M et al (2020) Enhancing therapeutic efficacy of donepezil by combined therapy: a comprehensive review. Curr Pharm Des 27:332–344. https://doi.org/10.2174/1381612826666201023144836
Takada-Takatori Y (2021) Donepezil reduces amyloid precursor protein endocytosis by resulting from increase in the expression of sorting nexin protein 33. Yakugaku Zasshi 141:851–856. https://doi.org/10.1248/YAKUSHI.20-00251-6
Tonstad S, Arons C, Rollema H et al (2020) Varenicline: mode of action, efficacy, safety and accumulated experience salient for clinical populations. Curr Med Res Opin 36:713–730. https://doi.org/10.1080/03007995.2020.1729708
Tan R, Bölükbaşi Hatip F, Açikalin Ö et al (2018) Effect of varenicline on behavioral deficits in a rat model of Parkinson’s disease induced by unilateral 6-hydroxydopamine lesion of substantia nigra. Behav Pharmacol 29:327–335. https://doi.org/10.1097/FBP.0000000000000355
Nemutlu Samur D, Akçay G, Yıldırım S et al (2022) Vortioxetine ameliorates motor and cognitive impairments in the rotenone-induced Parkinson’s disease via targeting TLR-2 mediated neuroinflammation. Neuropharmacology 208. https://doi.org/10.1016/J.NEUROPHARM.2022.108977
Chung ES, Bok E, Chung YC et al (2012) Cannabinoids prevent lipopolysaccharide-induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res 1451:110–116. https://doi.org/10.1016/J.BRAINRES.2012.02.058
Mücke M, Phillips T, Radbruch L et al (2018) Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane database Syst Rev 3. https://doi.org/10.1002/14651858.CD012182.PUB2
Ineichen BV, Moridi T, Granberg T, Piehl F (2020) Rituximab treatment for multiple sclerosis. Mult Scler 26:137–152. https://doi.org/10.1177/1352458519858604
Gonçalves CL, Vasconcelos FFP, Wessler LB et al (2020) Exposure to a high dose of amoxicillin causes behavioral changes and oxidative stress in young zebrafish. Metab Brain Dis 35:1407–1416. https://doi.org/10.1007/S11011-020-00610-6
Llorca PM, Fernandez JL (2007) Escitalopram in the treatment of major depressive disorder: clinical efficacy, tolerability and cost-effectiveness vs. venlafaxine extended-release formulation. Int J Clin Pract 61:702–710. https://doi.org/10.1111/j.1742-1241.2007.01335.x
Gizak A, Duda P, Pielka E et al (2020) GSK3 and miRNA in neural tissue: from brain development to neurodegenerative diseases. Biochim Biophys Acta – Mol Cell Res 1867
Gugliandolo A, Chiricosta L, Boccardi V et al (2020) Micrornas modulate the pathogenesis of Alzheimer’s disease: an in silico analysis in the human brain. Genes (Basel) 11:1–13. https://doi.org/10.3390/genes11090983
Chandrasekaran S, Bonchev D (2016) Network analysis of human post-mortem microarrays reveals novel genes, microRNAs, and mechanistic scenarios of potential importance in fighting Huntington’s disease. Comput Struct Biotechnol J 14:117–130. https://doi.org/10.1016/j.csbj.2016.02.001
Chan AWS, Kocerha J (2012) The path to microRNA therapeutics in psychiatric and neurodegenerative disorders. Front Genet 3. https://doi.org/10.3389/fgene.2012.00082
Hunsberger JG, Fessler EB, Chibane FL et al (2013) Mood stabilizer-regulated miRNAs in neuropsychiatric and neurodegenerative diseases: identifying associations and functions. Am J Transl Res 5(4):450
Fu CH, Han XY, Tong L et al (2021) miR-142 downregulation alleviates the impairment of spatial learning and memory, reduces the level of apoptosis, and upregulates the expression of pCaMKII and BAI3 in the hippocampus of APP/PS1 transgenic mice. Behav Brain Res 414. https://doi.org/10.1016/j.bbr.2021.113485
Li J, Li D, Huatao Z et al (2020) MicroRNA-338-5p alleviates neuronal apoptosis via directly targeting BCL2L11 in APP-PS1 mice 2020. Aging 12:20728–20742. https://doi.org/10.18632/aging.104005
Sun L, Zhang T, Xiu W et al (2020) MiR-107 overexpression attenuates neurotoxicity induced by 6-hydroxydopamine both in vitro and in vivo. Chem Biol Interact 315. https://doi.org/10.1016/j.cbi.2019.108908
Scarr E, Craig JM, Cairns MJ et al (2013) Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Transl Psychiatry 3. https://doi.org/10.1038/tp.2013.3
Tao Z, Feng C, Mao C et al (2019) MiR-4465 directly targets PTEN to inhibit AKT/mTOR pathway–mediated autophagy. Cell Stress Chaperones 24:105–113. https://doi.org/10.1007/s12192-018-0946-6
Shu Y, Qian J, Wang C (2020) Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients. Open Life Sci 15:647–653. https://doi.org/10.1515/biol-2020-0060
Fan C, Li Y, Lan T et al (2022) Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression - PubMed. Mol Ther 30:1300–1314. https://doi.org/10.1016/j.ymthe.2021.11.006
Liu CP, Zhong M, Sun JX et al (2021) miR-146a reduces depressive behavior by inhibiting microglial activation. Mol Med Rep 23. https://doi.org/10.3892/MMR.2021.12102
Oliveira SR, Dionísio PA, Gaspar MM et al (2021) miR-335 targets LRRK2 and mitigates inflammation in Parkinson’s disease. Front Cell Dev Biol 9. https://doi.org/10.3389/FCELL.2021.661461
Li X, Si W, Li Z et al (2021) miR-335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson’s disease. Int J Mol Med 47. https://doi.org/10.3892/ijmm.2021.4894
De Luna N, Turon-Sans J, Cortes-Vicente E et al (2020) Downregulation of miR-335-5P in amyotrophic lateral sclerosis can contribute to neuronal mitochondrial dysfunction and apoptosis. Sci Rep 10. https://doi.org/10.1038/s41598-020-61246-1
Li J, Meng H, Cao W, Qiu T (2015) MiR-335 is involved in major depression disorder and antidepressant treatment through targeting GRM4. Neurosci Lett 606:167–172. https://doi.org/10.1016/j.neulet.2015.08.038
Li L, Xu Y, Zhao M, Gao Z (2020) Neuro-protective roles of long non-coding RNA MALAT1 in Alzheimer’s disease with the involvement of the microRNA-30b/CNR1 network and the following PI3K/AKT activation. Exp Mol Pathol 104545:117. https://doi.org/10.1016/j.yexmp.2020.104545
Cai LJ, Tu L, Huang XM et al (2020) LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson’s disease. Mol Brain 13:130. https://doi.org/10.1186/s13041-020-00656-8
Fallah H, Azari I, Neishabouri SM et al (2019) Sex-specific up-regulation of lncRNAs in peripheral blood of patients with schizophrenia. Sci Rep 9. https://doi.org/10.1038/s41598-019-49265-z
Dong L-X, Zhang Y-Y, Bao H-L et al (2021) LncRNA NEAT1 promotes Alzheimer’s disease by down regulating micro-27a-3p. Am J Transl Res 13:8885–8896
Ke S, Yang Z, Yang F et al (2019) Long noncoding RNA NEAT1 aggravates Aβ-induced neuronal damage by targeting miR-107 in Alzheimer’s disease. Yonsei Med J 60:640–650. https://doi.org/10.3349/ymj.2019.60.7.640
Nishimoto Y, Nakagawa S, Okano H (2021) NEAT1 lncRNA and amyotrophic lateral sclerosis. Neurochem Int 105175:150. https://doi.org/10.1016/j.neuint.2021.105175
Katsel P, Roussos P, Fam P et al (2019) The expression of long noncoding RNA NEAT1 is reduced in schizophrenia and modulates oligodendrocytes transcription. npj Schizophr 5. https://doi.org/10.1038/s41537-019-0071-2
Fan Y, Zhao X, Lu K, Cheng G (2020) LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson’s disease via ablating microRNA-125b-5p. Brain Res Bull 157:119–127. https://doi.org/10.1016/j.brainresbull.2020.02.003
Ding Y, Luan W, Shen X et al (2022) LncRNA BDNF-AS as ceRNA regulates the miR-9-5p/BACE1 pathway affecting neurotoxicity in Alzheimer’s disease. Arch Gerontol Geriatr 99. https://doi.org/10.1016/j.archger.2021.104614
Guo CC, Hong JC, Gao ZM (2018) Silencing of LncRNA BDNF-AS attenuates Aβ25-35-induced neurotoxicity in PC12 cells by suppressing cell apoptosis and oxidative stress. Neurol Res 40:795–804. https://doi.org/10.1080/01616412.2018.1480921
Badrlou E, Ghafouri-Fard S, Omrani MD et al (2021) Expression of BDNF-associated lncRNAs in treatment-resistant schizophrenia patients. J Mol Neurosci 71:2249–2259. https://doi.org/10.1007/s12031-020-01772-9
Wang Y, Zhao X, Ju W et al (2015) Genome-wide differential expression of synaptic long noncoding RNAs in autism spectrum disorder. Transl Psychiatry 5. https://doi.org/10.1038/tp.2015.144
Zhou WY, Cai ZR, Liu J et al (2020) Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer 19
Altesha MA, Ni T, Khan A et al (2019) Circular RNA in cardiovascular disease. J Cell Physiol 234:5588–5600
Wang Z, Xu P, Chen B et al (2018) Identifying circRNA-associated-ceRNA networks in the hippocampus of Aß1-42-induced Alzheimer’s disease-like rats using microarray analysis. Aging (Albany NY) 10:775–788. https://doi.org/10.18632/aging.101427
Huang JL, Xu ZH, Yang SM et al (2018) Identification of differentially expressed profiles of Alzheimer’s disease associated circular RNAs in a Panax notoginseng saponins-treated Alzheimer’s disease mouse model. Comput Struct Biotechnol J 16:523–531. https://doi.org/10.1016/j.csbj.2018.10.010
Sun F, Zhang Y, Wu X et al (2022) Breviscapine combined with BMSCs reduces Aβ deposition in rat with Alzheimer’s disease by regulating circular RNA ciRS-7. Curr Mol Med 22. https://doi.org/10.2174/1566524022666220113151044
Zhang Y, Du L, Bai Y et al (2020) CircDYM ameliorates depressive-like behavior by targeting miR-9 to regulate microglial activation via HSP90 ubiquitination. Mol Psychiatry 25:1175–1190. https://doi.org/10.1038/s41380-018-0285-0
Kumar L, Shamsuzzama JP et al (2018) Functional characterization of novel circular RNA molecule, circzip-2 and its synthesizing gene zip-2 in C. elegans model of Parkinson’s disease. Mol Neurobiol 55:6914–6926. https://doi.org/10.1007/s12035-018-0903-5
Gillardon F, Mack M, Rist W et al (2008) MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteomics - Clin Appl 2:697–705. https://doi.org/10.1002/prca.200780025
Liu W, Zhang F, Zheng Y et al (2021) The role of circulating blood microRNA-374 and microRNA-10 levels in the pathogenesis and therapeutic mechanisms of major depressive disorder. Neurosci Lett 763. https://doi.org/10.1016/j.neulet.2021.136184
Sierksma A, Lu A, Salta E et al (2018) Deregulation of neuronal miRNAs induced by amyloid-β or TAU pathology 11 Medical and Health Sciences 1109 Neurosciences. Mol Neurodegener 13. https://doi.org/10.1186/s13024-018-0285-1
Han L, Tang Y, Bai X et al (2020) Association of the serum microRNA-29 family with cognitive impairment in Parkinson’s disease. Aging (Albany NY) 12:13518–13528. https://doi.org/10.18632/aging.103458
Li J, Chan MC, Yu Y et al (2017) MiR-29b contributes to multiple types of muscle atrophy. Nat Commun 8. https://doi.org/10.1038/ncomms15201
Pereira PA, Tomás JF, Queiroz JA et al (2016) Recombinant pre-miR-29b for Alzheimer’s disease therapeutics. Sci Rep 6. https://doi.org/10.1038/srep19946
Sarachana T, Zhou R, Chen G et al (2010) Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med 2. https://doi.org/10.1186/gm144
Thomas KT, Zakharenko SS (2021) MicroRNAs in the onset of schizophrenia. Cells 10
Wan YQ, Feng JG, Li M et al (2018) Prefrontal cortex miR-29b-3p plays a key role in the antidepressant-like effect of ketamine in rats. Exp Mol Med 50. https://doi.org/10.1038/s12276-018-0164-4
Lo Iacono L, Ielpo D, Parisi C et al (2021) MicroRNA-34a regulates 5-HT2C expression in dorsal raphe and contributes to the anti-depressant-like effect of fluoxetine. Neuropharmacology 190. https://doi.org/10.1016/j.neuropharm.2021.108559
Ma Q, Dasgupta C, Li Y et al (2016) Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis 89:202–212. https://doi.org/10.1016/j.nbd.2016.02.011
Moreau MP, Bruse SE, David-Rus R et al (2011) Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry 69:188–193. https://doi.org/10.1016/j.biopsych.2010.09.039
Nunomura A, Perry G (2020) RNA and oxidative stress in Alzheimer’s disease: focus on microRNAs. Oxid Med Cell Longev 2020
Watts ME, Williams SM, Nithianantharajah J, Claudianos C (2018) Hypoxia-induced microRNA-210 targets neurodegenerative pathways. Non-coding RNA 4. https://doi.org/10.3390/ncrna4020010
Piscopo P, Grasso M, Puopolo M et al (2018) Circulating miR-127-3p as a potential biomarker for differential diagnosis in frontotemporal dementia. J Alzheimer’s Dis 65:455–464. https://doi.org/10.3233/JAD-180364
Azevedo JA, Carter BS, Meng F et al (2016) The microRNA network is altered in anterior cingulate cortex of patients with unipolar and bipolar depression. J Psychiatr Res 82:58–67. https://doi.org/10.1016/j.jpsychires.2016.07.012
Fukuoka M, Takahashi M, Fujita H et al (2018) Supplemental treatment for Huntington’s disease with miR-132 that is deficient in Huntington’s disease brain. Mol Ther - Nucleic Acids 11:79–90. https://doi.org/10.1016/j.omtn.2018.01.007
Liu X, Wang H, Bei J et al (2021) The protective role of miR-132 targeting HMGA2 through the PI3K/AKT pathway in mice with Alzheimer’s disease. Am J Transl Res 13:4632–4643
Miller BH, Zeier Z, Xi L et al (2012) MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci U S A 109:3125–3130. https://doi.org/10.1073/pnas.1113793109
Boscher E, Goupil C, Petry S et al (2021) MicroRNA-138 overexpression alters Aβ42 levels and behavior in wildtype mice. Front Neurosci 14. https://doi.org/10.3389/fnins.2020.591138
Bridget M, Peplow P (2021) Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies. Neural Regen Res 16:2159–2169
Muiños-Gimeno M, Espinosa-Parrilla Y, Guidi M et al (2011) Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry 69:526–533. https://doi.org/10.1016/j.biopsych.2010.10.010
Zadehbagheri F, Hosseini E, Bagheri-Hosseinabadi Z et al (2019) Profiling of miRNAs in serum of children with attention-deficit hyperactivity disorder shows significant alterations. J Psychiatr Res 109:185–192. https://doi.org/10.1016/j.jpsychires.2018.12.013
Ghose J, Sinha M, Das E et al (2011) Regulation of miR-146a by RelA/NFkB and p53 in STHdh q111/Hdh q111 cells, a cell model of Huntington’s disease. PLoS One 6. https://doi.org/10.1371/journal.pone.0023837
Dinan TG (2010) MicroRNAs as a target for novel antipsychotics: a systematic review of an emerging field. Int J Neuropsychopharmacol 13:395–404
Rodriguez-Ortiz CJ, Prieto GA, Martini AC et al (2020) miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer’s disease. Aging Cell 19. https://doi.org/10.1111/acel.13118
Soliman R, Mousa NO, Rashed HR et al (2021) Assessment of diagnostic potential of some circulating microRNAs in amyotrophic lateral sclerosis patients, an Egyptian study. Clin Neurol Neurosurg 208. https://doi.org/10.1016/j.clineuro.2021.106883
Gou M, Pan S, Tong J et al (2021) Effects of microRNA-181b-5p on cognitive deficits in first-episode patients with schizophrenia: mediated by BCL-2. J Psychiatr Res 136:358–365. https://doi.org/10.1016/j.jpsychires.2021.02.003
Lu Y, Xu X, Dong R et al (2019) MicroRNA-181b-5p attenuates early postoperative cognitive dysfunction by suppressing hippocampal neuroinflammation in mice. Cytokine 120:41–53. https://doi.org/10.1016/j.cyto.2019.04.005
Sun Q, Wang S, Chen J et al (2019) MicroRNA-190 alleviates neuronal damage and inhibits neuroinflammation via Nlrp3 in MPTP-induced Parkinson’s disease mouse model. J Cell Physiol 234:23379–23387. https://doi.org/10.1002/jcp.28907
Yu Y, Cao XC (2019) MiR-190-5p in human diseases. Cancer Cell Int 19
Wang M, Li Z, Zuo Q (2020) miR-194-5p inhibits LPS-induced astrocytes activation by directly targeting neurexophilin 1. Mol Cell Biochem 471:203–213. https://doi.org/10.1007/s11010-020-03780-0
Wang T, Cheng Y, Han H et al (2019) MiR-194 accelerates apoptosis of Aβ1-42-transduced hippocampal neurons by inhibiting nrn1 and decreasing PI3K/Akt signaling pathway activity. Genes (Basel) 10. https://doi.org/10.3390/genes10040313
Li Y, Fan C, Gao R et al (2021) Hippocampal miR-211-5p regulates neurogenesis and depression-like behaviors in the rat. Neuropharmacology 194. https://doi.org/10.1016/j.neuropharm.2021.108618
Liu XH, Ning FB, Zhao DP et al (2021) Role of miR-211 in a PC12 cell model of Alzheimer’s disease via regulation of neurogenin 2. Exp Physiol 106:1061–1071. https://doi.org/10.1113/EP088953
Kim AH, Reimers M, Maher B et al (2010) MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res 124:183–191. https://doi.org/10.1016/j.schres.2010.07.002
Liu Y, Chang X, Hahn CG et al (2018) Non-coding RNA dysregulation in the amygdala region of schizophrenia patients contributes to the pathogenesis of the disease. Transl Psychiatry 8. https://doi.org/10.1038/s41398-017-0030-5
Stojanovic T, Benes H, Awad A et al (2021) Nicotine abolishes memory-related synaptic strengthening and promotes synaptic depression in the neurogenic dentate gyrus of miR-132/212 knockout mice. Addict Biol 26. https://doi.org/10.1111/adb.12905
Sun S, Han X, Li X et al (2018) MicroRNA-212-5p Prevents dopaminergic neuron death by inhibiting SIRT2 in MPTP-induced mouse model of Parkinson’s disease. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2018.00381
Wang Y, Chang Q (2020) MicroRNA miR-212 regulates PDCD4 to attenuate Aβ25–35-induced neurotoxicity via PI3K/AKT signaling pathway in Alzheimer’s disease. Biotechnol Lett 42:1789–1797. https://doi.org/10.1007/s10529-020-02915-z
Zhai X, Liu J, Ni A, Ye J (2020) MiR-497 promotes microglia activation and proinflammatory cytokines production in chronic unpredictable stress-induced depression via targeting FGF2. J Chem Neuroanat 110. https://doi.org/10.1016/j.jchemneu.2020.101872
Zhu L, Lin M, Ma J et al (2019) The role of LINC00094/miR-224-5p (miR-497-5p)/endophilin-1 axis in memantine mediated protective effects on blood-brain barrier in AD microenvironment. J Cell Mol Med 23:3280–3292. https://doi.org/10.1111/jcmm.14214
Zhu W, Zhang H, Gao J, Xu Y (2021) Silencing of miR-497-5p inhibits cell apoptosis and promotes autophagy in Parkinson’s disease by upregulation of FGF2. Environ Toxicol 36:2302–2312. https://doi.org/10.1002/tox.23344
Devon RS, Anderson S, Teague PW et al (2001) Identification of polymorphisms within disrupted in Schizophrenia 1 and disrupted in Schizophrenia 2, and an investigation of their association with schizophrenia and bipolar affective disorder. Psychiatr Genet 11:71–78. https://doi.org/10.1097/00041444-200106000-00003
King CA, Katz SH, Ghaziuddin N et al (1997) Diagnosis and assessment of depression and suicidality using the NIMH diagnostic interview schedule for children (DISC-2.3). J Abnorm Child Psychol 25:173–181. https://doi.org/10.1023/A:1025739730823
Tamizkar KH, Ghafouri-Fard S, Omrani MD et al (2021) Altered expression of lncRNAs in autism spectrum disorder. Metab Brain Dis 36:983–990. https://doi.org/10.1007/s11011-021-00681-z
Sunwoo JS, Lee ST, Im W et al (2017) Altered expression of the long noncoding RNA NEAT1 in Huntington’s disease. Mol Neurobiol 54:1577–1586. https://doi.org/10.1007/s12035-016-9928-9
Safari M, Noroozi R, Taheri M, Ghafouri-Fard S (2020) The rs12826786 in HOTAIR lncRNA is associated with risk of autism spectrum disorder. J Mol Neurosci 70:175–179. https://doi.org/10.1007/s12031-019-01421-w
Taheri M, Noroozi R, Sadeghpour S et al (2020) The rs4759314 SNP within Hotair lncRNA is associated with risk of multiple sclerosis. Mult Scler Relat Disord 101986:40. https://doi.org/10.1016/j.msard.2020.101986
Zhang Q, Huang XM, Liao JX et al (2021) LncRNA HOTAIR promotes neuronal damage through facilitating NLRP3 mediated-pyroptosis activation in Parkinson’s disease via regulation of miR-326/ELAVL1 axis. Cell Mol Neurobiol 41:1773–1786. https://doi.org/10.1007/s10571-020-00946-8
Zhao J, Li H, Chang N (2020) LncRNA hotair promotes MPP+-induced neuronal injury in Parkinson’s disease by regulating the miR-874-5p/ATG10 axis. EXCLI J 19:1141–1153. https://doi.org/10.17179/excli2020-2286
Quigley EMM (2017) Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep 17:1–9. https://doi.org/10.1007/S11910-017-0802-6/FIGURES/2
Acknowledgements
We would like to thank the senior management of Delhi Technological University for their constant support and guidance.
Author information
Authors and Affiliations
Contributions
The project is conceived by RG and PK. The data collected by RG, DA, and DY. The art work is done by RG and DA. The data were arranged and analyzed by all authors. The paper is written by RG, DA, DY, RKA, and PK. All authors have read the paper and expressed no objection to submit.
Corresponding author
Ethics declarations
Ethics Approval
This is not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Stress/anxiety-releasing factors, namely, serotonin, norepinephrine, and corticotrophins, in NPDs and NDDs
• Microbiota–gut–brain axis is a potential therapeutic target in the NPDs and NDDs
• Therapeutic role of γCaMKII and MAPK/ERK signaling in NPDs and NDDs
• Therapeutic considerations of post-translational modification in the pathophysiology of NPDs and NDDs
• Natural compounds, MTDL, and RNAs as therapeutic agents against NPDs and NDDs
• Potential involvement of repurposed drugs in the treatment of NPDs and NDDs
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, R., Advani, D., Yadav, D. et al. Dissecting the Relationship Between Neuropsychiatric and Neurodegenerative Disorders. Mol Neurobiol 60, 6476–6529 (2023). https://doi.org/10.1007/s12035-023-03502-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03502-9