Abstract
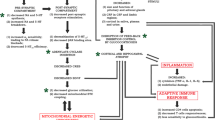
Temporal lobe epilepsy (TLE), accompanied by hippocampal sclerosis (HS), is the most common form of drug-resistant epilepsy (DRE). Nearly 20% of the patients showed seizure recurrence even after surgery, and the reasons are yet to be understood. Dysregulation of neurotransmitters is evident during seizures, which can induce excitotoxicity. The present study focused on understanding the molecular changes associated with Dopamine (DA) and glutamate signaling and their possible impact on the persistence of excitotoxicity and seizure recurrence in patients with drug-resistant TLE-HS who underwent surgery. According to the International League against Epilepsy (ILAE) suggested classification for seizure outcomes, the patients (n = 26) were classified as class 1 (no seizures) and class 2 (persistent seizures) using the latest post-surgery follow-up data to understand the prevalent molecular changes in seizure-free and seizure-recurrence patient groups. Our study uses thioflavin T assay, western blot analysis, immunofluorescence assays, and fluorescence resonance energy transfer (FRET) assays. We have observed a substantial increase in the DA and glutamate receptors that promote excitotoxicity. Patients who had seizure recurrence showed a significant increase in (pNR2B, p < 0.009; and pGluR1, p < 0.01), protein phosphatase1γ (PP1γ; p < 0.009), protein kinase A (PKAc; p < 0.001) and dopamine-cAMP regulated phospho protein32 (pDARPP32T34; p < 0.009) which are critical for long-term potentiation (LTP), excitotoxicity compared to seizure-free patients and controls. A significant increase in D1R downstream kinases like PKA (p < 0.001), pCAMKII (p < 0.009), and Fyn (p < 0.001) was observed in patient samples compared to controls. Anti-epileptic DA receptor D2R was found to be decreased in ILAE class 2 (p < 0.02) compared to class 1. Since upregulation of DA and glutamate signaling supports LTP and excitotoxicity, we believe it could impact seizure recurrence. Further studies about the impact of DA and glutamate signaling on the distribution of PP1γ at postsynaptic density and synaptic strength could help us understand the seizure microenvironment in patients.
Graphical Abstract
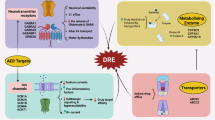
Dopamine, Glutamate signal crosstalk. Diagram representing the PP1γ regulation by NMDAR negative feedback inhibition signaling (green circle-left) and D1R signal (red circle-middle) domination over PP1γ though increased PKA, pDARPP32T34, and supports pGluR1, pNR2B in seizure recurrent patients. D1R-D2R hetero dimer activation (red circle-right) increases cellular Ca2+ and pCAMKIIα activation. All these events lead to calcium overload in HS patients and excitotoxicity, particularly in patients experiencing recurrent seizures.







Similar content being viewed by others
Data Availability
All the data provided in the manuscript and original data supplied as a supplementary file.
References
Berg AT, Shinnar S, Levy SR, Testa FM (1999) Childhood-onset epilepsy with and without preceding febrile seizures. Neurology 53:1742–1742. https://doi.org/10.1212/WNL.53.8.1742
Patterson KP, Baram TZ, Shinnar S (2014) Origins of Temporal Lobe Epilepsy: Febrile Seizures and Febrile Status Epilepticus. Neurotherapeutics 11:242–250. https://doi.org/10.1007/s13311-014-0263-4
Kim JA, Connors BW (2012) High temperatures alter physiological properties of pyramidal cells and inhibitory interneurons in hippocampus. Front Cell Neurosci 6. https://doi.org/10.3389/fncel.2012.00027
(1993) Guidelines for Epidemiologic Studies on Epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 34:592–596. https://doi.org/10.1111/j.1528-1157.1993.tb00433.x
Shinnar S, Hesdorffer DC, Nordli DR et al (2008) Phenomenology of prolonged febrile seizures: Results of the FEBSTAT study. Neurology 71:170–176. https://doi.org/10.1212/01.wnl.0000310774.01185.97
Wiebe S (2000) Epidemiology of Temporal Lobe Epilepsy. Can J Neurol Sci 27:S6–S10. https://doi.org/10.1017/S0317167100000561
Blair RDG (2012) Temporal Lobe Epilepsy Semiology. Epilepsy Res Treat 2012:1–10. https://doi.org/10.1155/2012/751510
Tatum WO (2012) Mesial Temporal Lobe Epilepsy. J Clin Neurophysiol 29:356–365. https://doi.org/10.1097/WNP.0b013e31826b3ab7
Dhikav V, Anand K (2012) Hippocampus in health and disease: An overview. Ann Indian Acad Neurol 15:239. https://doi.org/10.4103/0972-2327.104323
Gilbert PE, Brushfield AM (2009) The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry 33:774–781. https://doi.org/10.1016/j.pnpbp.2009.03.037
Zhao L, Nagao T, Desjardins GC et al (1994) Quantitative evaluation of neuronal loss in the dorsal hippocampus in rats with long-term pilocarpine seizures. Epilepsy Res 17:237–247. https://doi.org/10.1016/0920-1211(94)90054-X
Bozzi Y, Borrelli E (2013) The role of dopamine signaling in epileptogenesis. Front Cell Neurosci 7. https://doi.org/10.3389/fncel.2013.00157
Neve KA, Seamans JK, Trantham-Davidson H (2004) Dopamine Receptor Signaling. J Recept Signal Transduction 24:165–205. https://doi.org/10.1081/RRS-200029981
Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD (2010) Influence of Phasic and Tonic Dopamine Release on Receptor Activation. J Neurosci 30:14273–14283. https://doi.org/10.1523/JNEUROSCI.1894-10.2010
Klein MO, Battagello DS, Cardoso AR et al (2019) Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol Neurobiol 39:31–59. https://doi.org/10.1007/s10571-018-0632-3
Trantham-Davidson H (2004) Mechanisms Underlying Differential D1 versus D2 Dopamine Receptor Regulation of Inhibition in Prefrontal Cortex. J Neurosci 24:10652–10659. https://doi.org/10.1523/JNEUROSCI.3179-04.2004
Hallett PJ (2006) Dopamine D1 Activation Potentiates Striatal NMDA Receptors by Tyrosine Phosphorylation-Dependent Subunit Trafficking. J Neurosci 26:4690–4700. https://doi.org/10.1523/JNEUROSCI.0792-06.2006
Dunah AW, Sirianni AC, Fienberg AA et al (2004) Dopamine D1-Dependent Trafficking of Striatal N- Methyl-d-aspartate Glutamate Receptors Requires Fyn Protein Tyrosine Kinase but Not DARPP-32. Mol Pharmacol 65:121–129. https://doi.org/10.1124/mol.65.1.121
Yan J-Z, Xu Z, Ren S-Q et al (2011) Protein Kinase C Promotes N -Methyl-d-aspartate (NMDA) Receptor Trafficking by Indirectly Triggering Calcium/Calmodulin-dependent Protein Kinase II (CaMKII) Autophosphorylation. J Biol Chem 286:25187–25200. https://doi.org/10.1074/jbc.M110.192708
Moriguchi S, Shioda N, Han F et al (2009) Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus 19:844–854. https://doi.org/10.1002/hipo.20572
Snyder GL, Allen PB, Fienberg AA et al (2000) Regulation of Phosphorylation of the GluR1 AMPA Receptor in the Neostriatum by Dopamine and Psychostimulants In Vivo. J Neurosci 20:4480–4488. https://doi.org/10.1523/JNEUROSCI.20-12-04480.2000
Tukey DS, Ziff EB (2013) Ca 2+ -permeable AMPA (α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid) Receptors and Dopamine D1 Receptors Regulate GluA1 Trafficking in Striatal Neurons. J Biol Chem 288:35297–35306. https://doi.org/10.1074/jbc.M113.516690
Lee SP, So CH, Rashid AJ et al (2004) Dopamine D1 and D2 Receptor Co-activation Generates a Novel Phospholipase C-mediated Calcium Signal. J Biol Chem 279:35671–35678. https://doi.org/10.1074/jbc.M401923200
Perreault ML, Hasbi A, Alijaniaram M et al (2010) The Dopamine D1–D2 Receptor Heteromer Localizes in Dynorphin/Enkephalin Neurons. J Biol Chem 285:36625–36634. https://doi.org/10.1074/jbc.M110.159954
Perreault ML, Hasbi A, O’Dowd BF, George SR (2014) Heteromeric Dopamine Receptor Signaling Complexes: Emerging Neurobiology and Disease Relevance. Neuropsychopharmacology 39:156–168. https://doi.org/10.1038/npp.2013.148
Ng J, Rashid AJ, So CH et al (2010) Activation of calcium/calmodulin-dependent protein kinase IIα in the striatum by the heteromeric D1–D2 dopamine receptor complex. Neuroscience 165:535–541. https://doi.org/10.1016/j.neuroscience.2009.10.017
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276. https://doi.org/10.1002/neu.480230915
Barker-Haliski M, White HS (2015) Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb Perspect Med 5:a022863. https://doi.org/10.1101/cshperspect.a022863
Magnusson KR (1998) The aging of the NMDA receptor complex. Front Biosci 3:A368. https://doi.org/10.2741/A368
Henshall DC, Schindler CK, So NK et al (2004) Death-associated protein kinase expression in human temporal lobe epilepsy. Ann Neurol 55:485–494. https://doi.org/10.1002/ana.20001
Henshall DC, Clark RSB, Adelson PD et al (2000) Alterations in bcl-2 and caspase gene family protein expression in human temporal lobe epilepsy. Neurology 55:250–257. https://doi.org/10.1212/WNL.55.2.250
Foley K, McKee C, Nairn AC, Xia H (2021) Regulation of Synaptic Transmission and Plasticity by Protein Phosphatase 1. J Neurosci 41:3040–3050. https://doi.org/10.1523/JNEUROSCI.2026-20.2021
Beaulieu J-M, Gainetdinov RR (2011) The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol Rev 63:182–217. https://doi.org/10.1124/pr.110.002642
Amenta F, Mignini F, Ricci A et al (2001) Age-related changes of dopamine receptors in the rat hippocampus: a light microscope autoradiography study. Mech Ageing Dev 122:2071–2083. https://doi.org/10.1016/S0047-6374(01)00317-7
Seaman KL, Smith CT, Juarez EJ, et al (2019) Differential regional decline in dopamine receptor availability across adulthood: Linear and nonlinear effects of age. Hum Brain Mapp hbm.24585. https://doi.org/10.1002/hbm.24585
Jucaite A, Forssberg H, Karlsson P et al (2010) Age-related reduction in dopamine D1 receptors in the human brain: from late childhood to adulthood, a positron emission tomography study. Neuroscience 167:104–110. https://doi.org/10.1016/j.neuroscience.2010.01.034
Gramuntell Y, Klimczak P, Coviello S, et al (2021) Effects of Aging on the Structure and Expression of NMDA Receptors of Somatostatin Expressing Neurons in the Mouse Hippocampus. Front Aging Neurosci 13. https://doi.org/10.3389/fnagi.2021.782737
Konopka LM (2015) Near death experience: neuroscience perspective. Croat Med J 56:392–393. https://doi.org/10.3325/cmj.2015.56.392
Wieser HG, Blume WT, Fish D et al (2001) ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 42:282–286
Madhamanchi K, Madhamanchi P, Jayalakshmi S et al (2022) Endoplasmic reticulum stress and unfolded protein accumulation correlate to seizure recurrence in focal cortical dysplasia patients. Cell Stress Chaperones. https://doi.org/10.1007/s12192-022-01301-0
Beriault DR, Werstuck GH (2013) Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound. Biochim Biophysica Acta (BBA) – Mol Cell Res 1833:2293–2301. https://doi.org/10.1016/j.bbamcr.2013.05.020
Rahmati M, Taherabadi SJ (2021) The effects of exercise training on Kinesin and GAP-43 expression in skeletal muscle fibers of STZ-induced diabetic rats. Sci Rep 11:9535. https://doi.org/10.1038/s41598-021-89106-6
Bostani M, Rahmati M, Mard SA (2020) The effect of endurance training on levels of LINC complex proteins in skeletal muscle fibers of STZ-induced diabetic rats. Sci Rep 10:8738. https://doi.org/10.1038/s41598-020-65793-5
Rahmati M, Rashno A (2021) Automated image segmentation method to analyse skeletal muscle cross section in exercise-induced regenerating myofibers. Sci Rep 11:21327. https://doi.org/10.1038/s41598-021-00886-3
Cifelli P, Grace AA (2012) Pilocarpine-induced temporal lobe epilepsy in the rat is associated with increased dopamine neuron activity. Int J Neuropsychopharmacol 15:957–964. https://doi.org/10.1017/S1461145711001106
Gardoni F, Bellone C (2015) Modulation of the glutamatergic transmission by Dopamine: a focus on Parkinson, Huntington and Addiction diseases. Front Cell Neurosci 9. https://doi.org/10.3389/fncel.2015.00025
Bhattacharjee A, Kaczmarek L (2005) For K channels, Na is the new Ca. Trends Neurosci 28:422–428. https://doi.org/10.1016/j.tins.2005.06.003
Seeman P, Bzowej NH, Guan H-C et al (1987) Human brain dopamine receptors in children and aging adults. Synapse 1:399–404. https://doi.org/10.1002/syn.890010503
Rinne JO, Lönnberg P, Marjamäki P (1990) Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res 508:349–352. https://doi.org/10.1016/0006-8993(90)90423-9
Piggott MA, Perry EK, Perry RH, Court JA (1992) [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res 588:277–286. https://doi.org/10.1016/0006-8993(92)91586-4
Gasiorowska A, Wydrych M, Drapich P, et al (2021) The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front Aging Neurosci 13. https://doi.org/10.3389/fnagi.2021.654931
Ułas J, Cotman CW (1997) Decreased expression of N-methyl-d-aspartate receptor 1 messenger RNA in select regions of Alzheimer brain. Neuroscience 79:973–982. https://doi.org/10.1016/S0306-4522(97)00023-7
Jurado S (2018) AMPA Receptor Trafficking in Natural and Pathological Aging. Front Mol Neurosci 10. https://doi.org/10.3389/fnmol.2017.00446
Bernard C, Wheal HV (1995) Plasticity of AMPA and NMDA receptor-mediated epileptiform activity in a chronic model of temporal lobe epilepsy. Epilepsy Res 21:95–107. https://doi.org/10.1016/0920-1211(95)00017-5
Allen PB, Ouimet CC, Greengard P (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci 94:9956–9961https://doi.org/10.1073/pnas.94.18.9956
Connolly CN, Kittler JT, Thomas P et al (1999) Cell Surface Stability of γ-Aminobutyric Acid Type A Receptors. J Biol Chem 274:36565–36572. https://doi.org/10.1074/jbc.274.51.36565
Albert KA, Hemmings HC, Adamo AIB et al (2002) Evidence for Decreased DARPP-32 in the Prefrontal Cortex of Patients With Schizophrenia. Arch Gen Psychiatry 59:705. https://doi.org/10.1001/archpsyc.59.8.705
Picconi B, Centonze D, Håkansson K et al (2003) Loss of bidirectional striatal synaptic plasticity in L-DOPA–induced dyskinesia. Nat Neurosci 6:501–506. https://doi.org/10.1038/nn1040
Bibb JA, Nishi A, O’Callaghan JP et al (2001) Phosphorylation of Protein Phosphatase Inhibitor-1 by Cdk5. J Biol Chem 276:14490–14497. https://doi.org/10.1074/jbc.M007197200
Dixit AB, Banerjee J, Tripathi M et al (2017) Synaptic roles of cyclin-dependent kinase 5 & its implications in epilepsy. Indian J Med Res 145:179–188. https://doi.org/10.4103/ijmr.IJMR_1249_14
Maggio R, Aloisi G, Silvano E et al (2009) Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat Disord 15:S2–S7. https://doi.org/10.1016/S1353-8020(09)70826-0
Bao Y-N, Dai W-L, Fan J-F et al (2021) The dopamine D1–D2DR complex in the rat spinal cord promotes neuropathic pain by increasing neuronal excitability after chronic constriction injury. Exp Mol Med 53:235–249. https://doi.org/10.1038/s12276-021-00563-5
Rashid AJ, So CH, Kong MMC, et al (2007) D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of G q /11 in the striatum. Proc Natl Acad Sci 104:654–659https://doi.org/10.1073/pnas.0604049104
Kovac S, Dinkova Kostova AT, Herrmann AM, et al (2017) Metabolic and Homeostatic Changes in Seizures and Acquired Epilepsy-Mitochondria, Calcium Dynamics and Reactive Oxygen Species. Int J Mol Sci 18. https://doi.org/10.3390/ijms18091935
Samuels BA, Tsai L-H (2003) Cdk5 is a dynamo at the synapse. Nat Cell Biol 5:689–690. https://doi.org/10.1038/ncb0803-689
Chen B-S, Roche KW (2007) Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53:362–368. https://doi.org/10.1016/j.neuropharm.2007.05.018
Summers KC, Bogard AS, Tavalin SJ (2019) Preferential generation of Ca2+-permeable AMPA receptors by AKAP79-anchored protein kinase C proceeds via GluA1 subunit phosphorylation at Ser-831. J Biol Chem 294:5521–5535. https://doi.org/10.1074/jbc.RA118.004340
Prybylowski K, Chang K, Sans N et al (2005) The Synaptic Localization of NR2B-Containing NMDA Receptors Is Controlled by Interactions with PDZ Proteins and AP-2. Neuron 47:845–857. https://doi.org/10.1016/j.neuron.2005.08.016
Brown AM, Baucum AJ, Bass MA, Colbran RJ (2008) Association of Protein Phosphatase 1γ 1 with Spinophilin Suppresses Phosphatase Activity in a Parkinson Disease Model. J Biol Chem 283:14286–14294. https://doi.org/10.1074/jbc.M801377200
Hsieh-Wilson LC, Benfenati F, Snyder GL et al (2003) Phosphorylation of Spinophilin Modulates Its Interaction with Actin Filaments. J Biol Chem 278:1186–1194. https://doi.org/10.1074/jbc.M205754200
Acknowledgements
Human Brain Tissue Repository for Neurobiological Studies, Department of Neuropathology, National Institute of Mental Health and Neurosciences, Bangalore, India, provided the autopsied control brain samples for this study. Krishna Institute of Medical Sciences (KIMS), also the KIMS research foundation (KFRC), and Dr. Sailaja Department of Pathology, Krishna Institute of Medical Science, Secunderabad, India for technical assistance with data collection. The financial assistance from the Department of Science and Technology, Government of India, DST- SERB Core grant, file Nos. CRG/2020/005021, CRG/2019/002570, and financial support to the University of Hyderabad-IoE by the Ministry of Education, Government of India (F11/9/2019-U3 (A). DST-FIST, and UGC-SAP to the Department of Biotechnology and Bioinformatics, BUILDER-DBT-BT/INF/22/SP41176/2020 to the School of Life Sciences is gratefully acknowledged. Kishore Madamanchi is thankful to CSIR-UGC Fellowship under Ref. no: 22/12/2013(II)EU-V (341059).
Funding
The financial assistance to the lab from the Ministry of Science and Technology, Department of Science and Technology, Govt. of India, DST- SERB Core grant, file No. CRG/2020/005021; DST-CSRI files No. SR/CSRI/196/2016, CRG/2020/005021; Department of Biotechnology, Govt. of India, BT/PR18168/MED/29/1064/2016, BT/PR17686/MED/30/1664/ 2016, and financial support to the University of Hyderabad-IoE by the Ministry of Education, Govt. of India (F11/9/2019-U3 (A) DST-FIST and UGC-SAP for the department.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study concept. The work plan was designed by [Prof. Prakash Babu Phanithi]. Data collection and data processing performed by [Kishore Madhamanchi]. Tissue sample and data collection by [Pradeep Madhamanchi]. Epilepsy surgery, storage of patient samples, and individual patient data provided by [Manas Panigrahi, Sita Jayalakshmi, and Anuja Patil. The first draft of the manuscript was prepared by [Kishore Madhamanchi] Corresponding author, and all authors commented on the previous version. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Hyderabad IAEC/UH/151/2017/05/PPB/P13.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study.
Consent to Publish
For the current study, consent to publish has been received from all the patients.
Competing Interest
All the authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madhamanchi, K., Madhamanchi, P., Jayalakshmi, S. et al. Dopamine and Glutamate Crosstalk Worsen the Seizure Outcome in TLE-HS Patients. Mol Neurobiol 60, 4952–4965 (2023). https://doi.org/10.1007/s12035-023-03361-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03361-4