Abstract
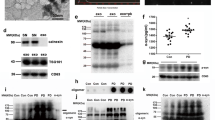
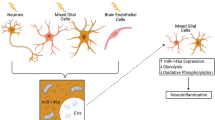
Inflammation is a common feature both for Parkinson’s disease (PD) and obesity-associated metabolic syndromes. Inflammation mediated by inflamed macrophages in white adipose tissue plays a pivotal role for the pathogenesis of metabolic syndromes. Exosomes are important carriers connecting peripheral tissues and the central nervous system (CNS). Therefore, we speculate that exosomes derived from inflamed macrophages may be involved in the pathological progression of PD. Here, we prepared exosomes from lipopolysaccharide (LPS) or interferon gamma (IFNγ) treated macrophages (inflamed macrophages) and examined their potential roles in PD. Our data showed that exosomes from inflamed macrophages stimulate proinflammatory cytokine expression in primary microglia and astrocytes. In vivo, inflamed macrophage exosomes induce behavioral defects in mice as evidenced by shortened duration in the rotarod test and prolonged latency in the pole test. The treatment of exosomes also reduces tyrosine hydroxylase (TH) positive cells in the substantia nigra pars compacta (SNpc) and striatum. All these PD-like phenotypes are likely due to the activation of microglia and astrocytes induced by exosomes from inflamed macrophages. Exosome sequencing, together with bioinformatics analysis and functional studies, revealed that exosomal miRNAs such as miR-155-5p are likely a key factor for inducing an inflammatory response in glial cells. These results indicate that exosomes derived from inflamed macrophages are likely a causative factor for developing PD. In this regard, inflamed macrophage exosomes might be a linker transducing the peripheral tissue inflammation into the CNS.









Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397(10291):2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. https://doi.org/10.1016/S1474-4422(16)30230-7
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. JAMA 323(6):548–560. https://doi.org/10.1001/jama.2019.22360
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934. https://doi.org/10.1016/j.cell.2010.02.016
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353(6301):777–783. https://doi.org/10.1126/science.aag2590
Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, Han B, Cho SJ, Yu JH, Park YG, Choi KM (2018) Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med 15(8):e1002640. https://doi.org/10.1371/journal.pmed.1002640
Cheong JLY, de Pablo-Fernandez E, Foltynie T, Noyce AJ (2020) The association between type 2 diabetes mellitus and Parkinson’s disease. J Parkinsons Dis 10(3):775–789. https://doi.org/10.3233/JPD-191900
Labandeira CM, Fraga-Bau A, Arias Ron D, Alvarez-Rodriguez E, Vicente-Alba P, Lago-Garma J, Rodriguez-Perez AI (2022) Parkinson’s disease and diabetes mellitus: common mechanisms and treatment repurposing. Neural Regen Res 17(8):1652–1658. https://doi.org/10.4103/1673-5374.332122
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148(5):852–871. https://doi.org/10.1016/j.cell.2012.02.017
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156(1–2):20–44. https://doi.org/10.1016/j.cell.2013.12.012
Sakers A, De Siqueira MK, Seale P, Villanueva CJ (2022) Adipose-tissue plasticity in health and disease. Cell 185(3):419–446. https://doi.org/10.1016/j.cell.2021.12.016
Lee YS, Wollam J, Olefsky JM (2018) An integrated view of immunometabolism. Cell 172(1–2):22–40. https://doi.org/10.1016/j.cell.2017.12.025
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478):eaau6977. https://doi.org/10.1126/science.aau6977
Pluchino S, Smith JA (2019) Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell 177(2):225–227. https://doi.org/10.1016/j.cell.2019.03.020
Kumari M, Anji A (2022) Small but mighty-exosomes, novel intercellular messengers in neurodegeneration. Biology (Basel) 11(3):413. https://doi.org/10.3390/biology11030413
Pinnell JR, Cui M, Tieu K (2021) Exosomes in Parkinson disease. J Neurochem 157(3):413–428. https://doi.org/10.1111/jnc.15288
Upadhya R, Shetty AK (2021) Extracellular vesicles for the diagnosis and treatment of Parkinson’s disease. Aging Dis 12(6):1438–1450. https://doi.org/10.14336/AD.2021.0516
Choi H, Choi K, Kim DH, Oh BK, Yim H, Jo S, Choi C (2022) Strategies for targeted delivery of exosomes to the brain: advantages and challenges. Pharmaceutics 14(3):672. https://doi.org/10.3390/pharmaceutics14030672
Floden AM, Li S, Combs CK (2005) Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J Neurosci 25(10):2566–2575. https://doi.org/10.1523/JNEUROSCI.4998-04.2005
Peng S, Wang C, Ma J, Jiang K, Jiang Y, Gu X, Sun C (2018) Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis in Parkinson’s disease models both in vitro and in vivo. Br J Pharmacol 175(4):631–643. https://doi.org/10.1111/bph.14110
Yang Y, Zhang S, Guan J, Jiang Y, Zhang J, Luo L (1868) Sun C (2022) SIRT1 attenuates neuroinflammation by deacetylating HSPA4 in a mouse model of Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis 5:166365. https://doi.org/10.1016/j.bbadis.2022.166365
Wang Y, Li L, Wu Y, Zhang S, Ju Q, Yang Y, Jin Y, Shi H, Sun C (2022) CD44 deficiency represses neuroinflammation and rescues dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res 177:106133. https://doi.org/10.1016/j.phrs.2022.106133
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57(6):563–581. https://doi.org/10.1016/s0301-0082(98)00069-0
Sun C, Wang M, Liu X, Luo L, Li K, Zhang S, Wang Y, Yang Y, Ding F, Gu X (2014) PCAF improves glucose homeostasis by suppressing the gluconeogenic activity of PGC-1alpha. Cell Rep 9(6):2250–2262. https://doi.org/10.1016/j.celrep.2014.11.029
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41(2–3):242–247. https://doi.org/10.1007/s12035-010-8105-9
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117(1):175–184. https://doi.org/10.1172/JCI29881
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233(9):6425–6440. https://doi.org/10.1002/jcp.26429
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830. https://doi.org/10.1172/JCI19451
Appari M, Channon KM, McNeill E (2018) Metabolic regulation of adipose tissue macrophage function in obesity and diabetes. Antioxid Redox Signal 29(3):297–312. https://doi.org/10.1089/ars.2017.7060
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171(2):372-384 e312. https://doi.org/10.1016/j.cell.2017.08.035
Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, Goldberg JL, He Z, Duan X, Bu G, Davis AA, Shekhar K, Torre A, Chan DC, Canto-Soler MV, Flanagan JG, Subramanian P, Rossi S, Brunner T, Bovenkamp DE, Calkins DJ (2022) Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener 17(1):23. https://doi.org/10.1186/s13024-022-00524-0
Su R, Sun M, Wang W, Zhang J, Zhang L, Zhen J, Qian Y, Zheng Y, Wang X (2017) A novel immunosuppressor, (5R)-5-hydroxytriptolide, alleviates movement disorder and neuroinflammation in a 6-OHDA hemiparkinsonian rat model. Aging Dis 8(1):31–43. https://doi.org/10.14336/AD.2016.0929
Fang X, Ma J, Mu D, Li B, Lian B, Sun C (2020) FGF21 Protects dopaminergic neurons in Parkinson’s disease models via repression of neuroinflammation. Neurotox Res 37(3):616–627. https://doi.org/10.1007/s12640-019-00151-6
Li C, Qin S, Wen Y, Zhao W, Huang Y, Liu J (2022) Overcoming the blood-brain barrier: exosomes as theranostic nanocarriers for precision neuroimaging. J Control Release 349:902–916. https://doi.org/10.1016/j.jconrel.2022.08.002
Morad G, Carman CV, Hagedorn EJ, Perlin JR, Zon LI, Mustafaoglu N, Park TE, Ingber DE, Daisy CC, Moses MA (2019) Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano 13(12):13853–13865. https://doi.org/10.1021/acsnano.9b04397
Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, Shi M, Banks WA, Zhang J (2017) Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun 5(1):71. https://doi.org/10.1186/s40478-017-0470-4
Liu L, Guo H, Song A, Huang J, Zhang Y, Jin S, Li S, Zhang L, Yang C, Yang P (2020) Progranulin inhibits LPS-induced macrophage M1 polarization via NF-small ka CyrillicB and MAPK pathways. BMC Immunol 21(1):32. https://doi.org/10.1186/s12865-020-00355-y
Huang R, Wang X, Zhou Y, Xiao Y (2017) RANKL-induced M1 macrophages are involved in bone formation. Bone Res 5:17019. https://doi.org/10.1038/boneres.2017.19
Bandow K, Hasegawa H, Tomomura M, Tomomura A (2020) Caldecrin inhibits lipopolysaccharide-induced pro-inflammatory cytokines and M1 macrophage polarization through the immunoreceptor triggering receptor expressed in myeloid cells-2. Biochem Biophys Res Commun 523(4):1027–1033. https://doi.org/10.1016/j.bbrc.2020.01.045
Isaksson R, Casselbrant A, Elebring E, Hallberg M, Larhed M, Fandriks L (2020) Direct stimulation of angiotensin II type 2 receptor reduces nitric oxide production in lipopolysaccharide treated mouse macrophages. Eur J Pharmacol 868:172855. https://doi.org/10.1016/j.ejphar.2019.172855
Cuomo-Haymour N, Bergamini G, Russo G, Kulic L, Knuesel I, Martin R, Huss A, Tumani H, Otto M, Pryce CR (2022) Differential expression of serum extracellular vesicle miRNAs in multiple sclerosis: disease-stage specificity and relevance to pathophysiology. Int J Mol Sci 23(3):1664. https://doi.org/10.3390/ijms23031664
Bokobza C, Joshi P, Schang AL, Csaba Z, Faivre V, Montane A, Galland A, Benmamar-Badel A, Bosher E, Lebon S, Schwendimann L, Mani S, Dournaud P, Besson V, Fleiss B, Gressens P, Van Steenwinckel J (2022) miR-146b protects the perinatal brain against microglia-induced hypomyelination. Ann Neurol 91(1):48–65. https://doi.org/10.1002/ana.26263
Ma Q, Zhao H, Tao Z, Wang R, Liu P, Han Z, Ma S, Luo Y, Jia J (2016) MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging Dis 7(6):705–714. https://doi.org/10.14336/AD.2016.0320
Pan Z, Zhu LJ, Li YQ, Hao LY, Yin C, Yang JX, Guo Y, Zhang S, Hua L, Xue ZY, Zhang H, Cao JL (2014) Epigenetic modification of spinal miR-219 expression regulates chronic inflammation pain by targeting CaMKIIgamma. J Neurosci 34(29):9476–9483. https://doi.org/10.1523/JNEUROSCI.5346-13.2014
Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der PouwKraan TC, van Zonneveld AJ, Horrevoets AJ, Prat A, Romero IA, de Vries HE (2013) MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J Neurosci 33(16):6857–6863. https://doi.org/10.1523/JNEUROSCI.3965-12.2013
Woodbury ME, Freilich RW, Cheng CJ, Asai H, Ikezu S, Boucher JD, Slack F, Ikezu T (2015) miR-155 Is Essential for inflammation-induced hippocampal neurogenic dysfunction. J Neurosci 35(26):9764–9781. https://doi.org/10.1523/JNEUROSCI.4790-14.2015
Caggiu E, Paulus K, Mameli G, Arru G, Sechi GP, Sechi LA (2018) Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci 13:1–4. https://doi.org/10.1016/j.ensci.2018.09.002
Thome AD, Harms AS, Volpicelli-Daley LA, Standaert DG (2016) microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of parkinson disease. J Neurosci 36(8):2383–2390. https://doi.org/10.1523/JNEUROSCI.3900-15.2016
Gong X, Huang M, Chen L (2022) Mechanism of miR-132–3p promoting neuroinflammation and dopaminergic neurodegeneration in Parkinson’s disease. eNeuro 9(1):ENEURO.0393-21.2021. https://doi.org/10.1523/ENEURO.0393-21.2021
Han S, Lin F, Ruan Y, Zhao S, Yuan R, Ning J, Jiang K, Xie J, Li H, Li C, Rao T, Yu W, Xia Y, Zhou X, Cheng F (2021) miR-132-3p promotes the cisplatin-induced apoptosis and inflammatory response of renal tubular epithelial cells by targeting SIRT1 via the NF-kappaB pathway. Int Immunopharmacol 99:108022. https://doi.org/10.1016/j.intimp.2021.108022
Peng S, Yan Y, Li R, Dai H, Xu J (2021) Extracellular vesicles from M1-polarized macrophages promote inflammation in the temporomandibular joint via miR-1246 activation of the Wnt/beta-catenin pathway. Ann N Y Acad Sci 1503(1):48–59. https://doi.org/10.1111/nyas.14590
Li G, Wang B, Ding X, Zhang X, Tang J, Lin H (2021) Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp Mol Med 53(7):1180–1191. https://doi.org/10.1038/s12276-021-00651-6
Zhang K, Feng Y, Liang Y, Wu W, Chang C, Chen D, Chen S, Gao J, Chen G, Yi L, Cheng D, Zhen G (2021) Epithelial miR-206 targets CD39/extracellular ATP to upregulate airway IL-25 and TSLP in type 2-high asthma. JCI Insight 6(11):e148103. https://doi.org/10.1172/jci.insight.148103
Ravanidis S, Bougea A, Papagiannakis N, Koros C, Simitsi AM, Pachi I, Breza M, Stefanis L, Doxakis E (2020) Validation of differentially expressed brain-enriched microRNAs in the plasma of PD patients. Ann Clin Transl Neurol 7(9):1594–1607. https://doi.org/10.1002/acn3.51146
Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M (2014) Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A 111(41):14888–14893. https://doi.org/10.1073/pnas.1408301111
Funding
This work was provided by the National Key Research and Development Program of China (2017YFA0701304); the National Natural Science Foundation of China (81970747; 32271193); the Scientific Program of Changshu City (CS202236); the Scientific Program of Nantong City (MS22021010; MS22019005); the Nantong Science and Technology Foundation of China (JC2021058); and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Y. J., R. W., and L. L. performed the experiments and analyzed the data. L. S. analyzed the data. Y. G. and C. S. designed the study. Y. J. and C. S. wrote the manuscript. C. S. supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Jin and Runze Wu contributed equally.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, Y., Wu, R., Li, L. et al. Exosomes from Inflamed Macrophages Promote the Progression of Parkinson’s Disease by Inducing Neuroinflammation. Mol Neurobiol 60, 1914–1928 (2023). https://doi.org/10.1007/s12035-022-03179-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03179-6