Abstract
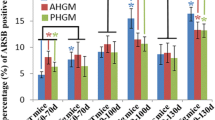
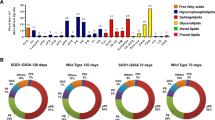
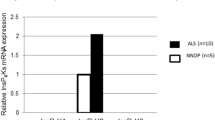
The pathogenesis of amyotrophic lateral sclerosis (ALS) might exist some relationships with the abnormal lipidomic metabolisms. Therefore, we observed and analyzed the alteration of perilipin 4 (PLIN 4) distribution in the anterior horns (AH); the central canals (CC) and its surrounding gray matter; the posterior horns (PH); and the anterior, lateral, and posterior funiculus (AF, LF, and PF) of the cervical, thoracic, and lumbar segments, as well as the alteration of PLIN 4 expression in the entire spinal cords at the pre-onset, onset, and progression stages of Tg(SOD1*G93A)1Gur (TG) mice and the same period of wild-type(WT) by fluorescent immunohistochemistry, the Western blot, and the image analysis. Results showed that the PLIN 4 distributions in the spinal AH, CC and its surrounding gray matter, PH, AF, and PF of the cervical, thoracic, and lumbar segments in the TG mice at the pre-onset, onset, and progression stages significantly increased compared with those at the same periods of WT mice; the gray matter was especially significant. No significant changes were detected in the LF. PLIN 4 extensively distributed in the neurons and the proliferation neural cells. The PLIN 4 distributions significantly gradually increased from the pre-onset to onset to progression stages, and significantly correlated with the gradual increase death of neural cells. Total PLIN 4 expression in the spinal cords of TG mice significantly increased from the pre-onset, to onset, and to progression stages compared with that in the WT mice. Our data suggested that the PLIN 4 distribution and expression alterations might participate in the death of neural cells in the pathogenesis of ALS through modulating the lipidomic metabolisms and the neural cell proliferation.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Cermelli S, Guo Y, Gross SP, Welte MA (2006) The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol 16(18):1783–1795. https://doi.org/10.1016/j.cub.2006.07.062
Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H et al (2011) The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 121:2102–2110. https://doi.org/10.1172/JCI46069
Palmer JWTB, Hoppel CL (1986) Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol 250(5 Pt 2):H741–H748. https://doi.org/10.1111/j.1748-1716.1986.tb07884.x
Li Y, Lee S, Langleite T, Norheim F, Pourteymour S, Jensen J, Stadheim HK, Storås TH et al (2014) Subsarcolemmal lipid droplet responses to a combined endurance and strength exercise intervention. Physiol Rep 2(11):e12187. https://doi.org/10.14814/phy2.12187
Zhang H, Wang Y, Li J, Yu J, Pu J, Li L, Zhang H, Zhang S et al (2011) Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J Proteome Res 10(10):4757–4768. https://doi.org/10.1021/pr200553c
Na H, Zhang P, Ding Y, Yang L, Wang Y, Zhang H, Xie Z, Yang F et al (2013) Proteomic studies of isolated lipid droplets from bacteria, C. elegans, and mammals. Methods Cell Biol 116:1–14. https://doi.org/10.1016/B978-0-12-408051-5.00001-2
Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C (2010) Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51(3):468–471. https://doi.org/10.1194/jlr.R000034
Brasaemle DL (2007) Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48(12):2547–2559. https://doi.org/10.1194/jlr.R700014-JLR200
Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplet. J Biol Chem 266(17):11341–11346
DA Servetnick BD, Gruia-Gray J, Kimmel AR, Wolff J, Londos C (1995) Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J Biol Chem 270(28):16970–16973. https://doi.org/10.1074/jbc.270.28.16970
Dalen KT, Schoonjans K, Ulven SM, Weedon-Fekjaer MS, Bentzen TG, Koutnikova H, Auwerx J, Nebb HI (2004) Associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes 53(5):1243–1252. https://doi.org/10.2337/diabetes.53.5.1243
Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE (2003) Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem 278(39):37713–37721. https://doi.org/10.1074/jbc.M304025200
Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A et al (2006)OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55(12):3418–3428. https://doi.org/10.2337/db06-0399
Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T (2006) MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem 281(20):14232–14240. https://doi.org/10.1074/jbc.M601682200
Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI (2007) LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta 1771(2):210–227. https://doi.org/10.1016/j.bbalip.2006.11.011
Bindesboll C, Berg O, Arntsen B, Nebb HI (2013) Dalen KT. Fatty acids regulate perilipin5 in muscle by activating PPAR delta. J Lipid Res 54(7):1949–1963. https://doi.org/10.1194/jlr.M038992
Pourteymour S, Lee S, Langleite TM, Eckardt K, Hjorth M, Bindesbøll C, Dalen KT, Birkeland KI et al (2015) Perilipin 4 in human skeletal muscle: localization and effect of physical activity. Physiol Rep 3(8):e12481. https://doi.org/10.14814/phy2.12481
Chaves-Filho AB, Pinto IFD, Dantas LS, Xavier AM, Inague A, Faria RL, Medeiros MHG, Glezer I et al (2019) Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci Rep 9(1):11642. https://doi.org/10.1038/s41598-019-48059-7
Jiménez-Riani M, Díaz-Amarilla P, Isasi E, Casanova G, Barbeito L, Olivera-Bravo S (2017) Ultrastructural features of aberrant glial cells isolated from the spinal cord of paralytic rats expressing the amyotrophic lateral sclerosis-linked SOD1G93A mutation. Cell Tissue Res 370(3):391–401. https://doi.org/10.1007/s00441-017-2681-1
Schifer D, Cordera S, Cavalla P, Migheli A (1996) Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J Neurol Sci 139(Suppl):27–33. https://doi.org/10.1016/0022-510X(96)00073-1
Nagy D, Kato T, Kushner PD (1994) Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res 38(3):336–347. https://doi.org/10.1002/jnr.490380312
Vargas MR, Johnson JA (2010) Astrogliosis in amyotrophic lateral sclerosis: role and therapeutic potential of astrocytes. Neurotherapeutics 7(4):471–481. https://doi.org/10.1016/j.nurt.2010.05.012
Zhang J, Huang P, Wu C, Liang H, Li Y, Zhu L, Lu Y, Tang C et al (2018) Preliminary observation about alteration of proteins and their potential functions in spinal cord of SOD1 G93A transgenic mice. Int J Biol Sci 14(10):1306–1320. https://doi.org/10.7150/ijbs.26829
Liang H, Wu C, Deng Y, Zhu L, Zhang J, Gan W, Tang C, Xu R (2017) Aldehyde dehydrogenases 1A2 expression and distribution are potentially associated with neuron death in spinal cord of Tg(SOD1*G93A)1Gur mice. Int J Biol Sci 13(5):574–587. https://doi.org/10.7150/ijbs.19150
Zhou Q, Zhu L, Qiu W, Liu Y, Yang F, Chen W, Xu R (2020) Nicotinamide riboside enhances mitochondrial proteostasis and adult neurogenesis through activation of mitochondrial unfolded protein response signaling in the brain of ALS SOD1 mice. Int J Biol Sci 16(2):284–297. https://doi.org/10.7150/ijbs.38487
Ludolph AC, Bendotti C, Blaugrund E, Chio A, Greensmith L, Loeffler JP, Mead R, Niessen HG et al (2010) Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph Lateral Scler 11(1-2):38–45. https://doi.org/10.3109/17482960903545334
Adibhatla RM, Hatcher JF (2007) Role of lipids in brain injury and diseases. Future Lipidol 2(4):403–422. https://doi.org/10.2217/17460875.2.4.403
Schmitt F, Hussain G, Dupuis L, Loeffler JP, Henriques A (2014) A plural role for lipids in motor neuron diseases: energy, signaling and structure. Front Cell Neurosci 8:25. https://doi.org/10.3389/fncel.2014.00025
Zhang Y, Appelkvist EL, Kristensson K, Dallner G (1996) The lipid compositions of different regions of rat brain during development and aging. Neurobiol Aging 17(6):869–875. https://doi.org/10.1016/S0197-4580(96)00076-0
Poon HF, Calabrese V, Scapagnini G, Butterfield DA (2004) Free radicals and brain aging. Clin Geriatr Med 20(2):329–359
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Sato N, Morishita R (2015) The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci 7:119. https://doi.org/10.3389/fnagi.2015.00199
Taylor JP, Brown RH, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539(7628):197–206. https://doi.org/10.1038/nature20413
Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z et al (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Prim 3:17071. https://doi.org/10.1038/nrdp.2017.71
Parakh S, Spencer DM, Halloran MA, Soo KY, Atkin JD (2013) Redox regulation in amyotrophic lateral sclerosis. Oxid Med Cell Longev 2013:408681. https://doi.org/10.1155/2013/408681
Vandoorne T, De Bock K, Van Den Bosch L (2018) Energy metabolism in ALS: an underappreciated opportunity? Acta Neuropathol 135(361-374):489–509. https://doi.org/10.1007/s00401-018-1835-x
Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP (2002) Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress - induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol 52(4):448–457. https://doi.org/10.1002/ana.10312
Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci 101(7):2070–2075. https://doi.org/10.1073/pnas.0305799101
Dodge JC, Treleaven CM, Pacheco J, Cooper S, Bao C, Cromwell M, Sardi SP, Chuang WL et al (2015) Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc Natl Acad Sci 112(26):8100–8105. https://doi.org/10.1073/pnas.1508767112
Henriques A, Croixmarie V, Priestman DA, Rosenbohm A, Dirrig-Grosch S, D’Ambra E, Huebecke M, HUSSAIN G et al (2015) Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum Mol Genet 24(25):7390–7405. https://doi.org/10.1093/hmg/ddv439
Henriques A, Croixmarie V, Bouscary A, Mosbach A, Keime C, Boursier-Neyret C, Walter B, Spedding M et al (2018) Sphingolipid metabolism is dysregulated at transcriptomic and metabolic levels in the spinal cord of an animal model of amyotrophic lateral sclerosis. Front Mol Neurosci 10:433. https://doi.org/10.3389/fnmol.2017.00433
Blasco H, Veyrat-Durebex C, Bocca C, Patin F, Vourc’h P, Kouassi Nzoughet J, Lenaers G, Andres CR et al (2017) Lipidomics reveals cerebrospinal-fluid signatures of ALS. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-17389-9
Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST (2018) Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci 11:10. https://doi.org/10.3389/fnmol.2018.00010
Veyrat-Durebex C, Bris C, Codron P, Bocca C, Chupin S, Corcia P, Vourc’h P, Hergesheimer R et al (2019)Metabo-lipidomics of fibroblasts and mitochondrial-endoplasmic reticulum extracts from ALS patients shows alterations in purine, pyrimidine, energetic, and phospholipid metabolisms. Mol Neurobiol 56:5780–5791. https://doi.org/10.1007/s12035-019-1484-7
Čopič A, Antoine-Bally S, Giménez-Andrés M, La Torre GC, Antonny B, Manni MM, Pagnotta S, Guihotet J et al (2018) A giant amphipathic helix from a perilipin that is adapted for coating lipid droplets. Nat Commun 9(1):1332. https://doi.org/10.1038/s41467-018-03717-8
Chen W, Chang B, Wu X, Li L, Sleeman M, Chan L (2013) Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am J Physiol Endocrinol Metab 304(7):E770–E779. https://doi.org/10.1152/ajpendo.00523.2012
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (30560042, 81160161, and 81360198), the Education Department of Jiangxi Province (GJJ170042, GJJ13198, GJJ170021), the Jiangxi Provincial Department of Science and Technology ([2014]-47, 20142BBG70062, 20171BAB215022), and the Health and Family Planning Commission of Jiangxi province (20181019) for supporting this study.
Funding
This research was supported by the National Natural Science Foundation of China (30560042, 81160161, and 81360198), the Education Department of Jiangxi Province (GJJ170042, GJJ13198, GJJ170021), the Jiangxi Provincial Department of Science and Technology ([2014]-47, 20142BBG70062, 20171BAB215022), and the Health and Family Planning Commission of Jiangxi province (20181019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal experiments were approved by the Animal Experiment Committee of the Animal Management Committee of Jiangxi Provincial People’s Hospital and Affiliated People’s Hospital of Nanchang University and were performed in accordance with the Guidelines for Animal Experiments at the Animal Management Committee of Jiangxi Provincial People’s Hospital and Affiliated People’s Hospital of Nanchang University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, L., Hu, F., Li, C. et al. Perilipin 4 Protein: an Impending Target for Amyotrophic Lateral Sclerosis. Mol Neurobiol 58, 1723–1737 (2021). https://doi.org/10.1007/s12035-020-02217-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02217-5