Abstract
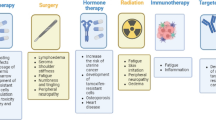
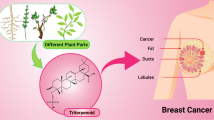
Breast cancer (BC) is a highly debilitating malignancy affecting females globally and imposing a substantial burden on healthcare systems in both developed and developing nations. Despite the application of conventional therapeutic modalities such as chemotherapy, radiation therapy, and hormonal intervention, BC frequently exhibits resistance, necessitating the urgent development of novel, cost-effective, and accessible treatment strategies. In this context, there is a growing scientific interest in exploring the pharmacological potential of chemical compounds derived from botanical sources, which often exhibit notable biological activity. Extensive in vitro and in vivo investigations have revealed the capacity of these compounds, referred to as phytochemicals, to attenuate the metastatic cascade and reduce the risk of cancer dissemination. These phytochemicals exert their effects through modulation of key molecular and metabolic processes, including regulation of the cell cycle, induction of apoptotic cell death, inhibition of angiogenesis, and suppression of metastatic progression. To shed light on the latest advancements in this field, a comprehensive review of the scientific literature has been conducted, focusing on secondary metabolite agents that have recently been investigated and have demonstrated promising anticancer properties. This review aims to delineate their underlying mechanisms of action and elucidate the associated signaling pathways, thereby contributing to a deeper understanding of their therapeutic potential in the context of BC management.





Similar content being viewed by others
Data Availability
There is no data to be disclosed separately for the current review article.
Abbreviations
- MCF-7:
-
Michigan Cancer Foundation-7
- T47D:
-
Differentiated epithelial substrain
- MDA-MB-453:
-
MD Anderson-Metastatic Breast-453
- Bcl-2:
-
B-cell lymphoma 2
- VEGF:
-
Vascular endothelial growth factor
- FOXM1-ER-α:
-
Forkhead box M1 Estrogen receptor α
- EMT:
-
Epithelial–mesenchymal transition
- PTEN:
-
Phosphatase and tensin homolog
- CDH1:
-
E-cadherin
- CD44:
-
Cluster of differentiation 4
- Akt:
-
Ak strain transforming
- SP1:
-
Specificity protein1
- Cav-1:
-
Caveolin-1;
- P-gp:
-
P-glycoprotein
- hTERT:
-
Human Telomerase Reverse Transcriptase
- NF-κB:
-
Nuclear factor-κB
- CDK2:
-
Cyclin-dependent kinase 2
- ZER:
-
Zerumbone
- CXCR4:
-
C-X-C motif chemokine receptor 4
- IC50 :
-
Inhibitory concentration
- McTN:
-
Microtentacles
- RUNX2:
-
Runt-related transcription factor 2
- CDK6:
-
Cyclin-dependent kinase 6
- ATF-3:
-
Activating transcription factor 3
- FBI‐1:
-
Factor that binds to inducer of short transcripts‐1
- mTOR:
-
Mammalian target of rapamycin
- LOX:
-
Lysyl oxidase
- MDM2:
-
Mouse double minute 2 homolog
- BRCA1:
-
Breast Cancer gene 1
- p21Cip1:
-
Cyclin-dependent kinase inhibitor p21
- EphA2:
-
Ephrin type-A receptor 2
- Twist1:
-
Twist-related protein 1
- STAT3:
-
Signal transducer and activator of transcription 3
- CCL2:
-
CC-Chemokine ligand 2
- POLD1:
-
Polymerase δ catalytic subunit gene 1
- JAK/STAT:
-
Janus kinase/signal transducers and activators of transcription
- MAP kinase:
-
Mitogen-activated protein kinase
- BNIP3:
-
Bcl-2 interacting protein 3
- BIRC5:
-
Baculoviral IAP Repeat Containing 5
- TNFα:
-
Tumor necrosis factor α
- GADD45A:
-
Growth arrest and DNA damage-inducible 45 alpha
- HRK:
-
Harakiri
- TNFRSF25:
-
TNF receptor superfamily member 25
- TNFRSF11B:
-
Tumor necrosis factor receptor superfamily member 11B
- TP53:
-
Tumor protein P53
- GM-CSF:
-
Granulocyte macrophage-colony-stimulating factor
- TSLP:
-
Thymic stromal lymphopoietin
- 4EBP1:
-
4E-binding protein 1
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- MSH2:
-
MutS homolog 2
- MGMT:
-
O6-Methylguanine-DNA methyltransferase
- XPC:
-
Xeroderma pigmentosum complementation gene
- OGG1:
-
8-Oxoguanine DNA glycosylase
- H3K9:
-
Histone H3 lysine 9
- TXNRD2:
-
Thioredoxin reductase 2
- HIF-1:
-
Hypoxia-inducible factor 1
- GPR30 :
-
G Protein-Coupled Receptor
- HOTAIR:
-
HOX transcript antisense intergenic RNA
- TXNIP:
-
Thioredoxin-interacting protein
- LC3-II:
-
LC3 phosphatidylethanolamine conjugate
References
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:59. https://doi.org/10.1186/s12964-020-0530-4.
Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2020;126:2225–49.
Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Alam S, Kelleher SL (2012) Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 4:875
Jin X, Mu P. Targeting breast cancer metastasis. Breast Cancer Basic Clin Res. 2015. https://doi.org/10.4137/BCBCR.S25460.
Fedele M, Cerchia L, Chiappetta G. The epithelial-to-mesenchymal transition in breast cancer: Focus on basal-like carcinomas. Cancers (Basel). 2017. 9: 134
Yeo SK, Guan JL. Breast cancer: multiple subtypes within a tumor? Trends Cancer. 2017. https://doi.org/10.1016/j.trecan.2017.09.001.
Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274:113–26.
Kitsios K, Sharifi S, Mahmoudi M. Nanomedicine technologies for diagnosis and treatment of breast cancer. ACS Pharmacol. Transl. Sci. 2023.
Tilsed CM, Fisher SA, Nowak AK, Lake RA, Lesterhuis WJ. Cancer chemotherapy: insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022.
Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7.
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106.
Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.16723.
Sharifi-Rad J, Ozleyen A, Tumer TB, Adetunji CO, El ON, Balahbib A, et al. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules. 2019. https://doi.org/10.3390/biom9110679.
Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68:153–65.
Gahtori R, Tripathi AH, Kumari A, Negi N, Paliwal A, Tripathi P, et al. Anticancer plant-derivatives: deciphering their oncopreventive and therapeutic potential in molecular terms. Futur J Pharm Sci. 2023;9:14.
Yin L, Duan J-J, Bian X-W, Yu S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. https://doi.org/10.1186/s13058-020-01296-5.
Dewi C, Fristiohady A, Amalia R, Khairul Ikram NK, Ibrahim S, Muchtaridi M. Signaling pathways and natural compounds in triple-negative breast cancer cell line. Molecules. 2022;27:3661.
Shindikar A, Singh A, Nobre M, Kirolikar S. Curcumin and resveratrol as promising natural remedies with nanomedicine approach for the effective treatment of triple negative breast cancer. J Oncol. 2016;2016:1–13.
Li X-X, Liu C, Dong S-L, Ou C-S, Lu J-L, Ye J-H, et al. Anticarcinogenic potentials of tea catechins. Front Nutr. 2022. https://doi.org/10.3389/fnut.2022.1060783/full.
Mazurakova A, Koklesova L, Samec M, Kudela E, Kajo K, Skuciova V, et al. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J. 2022;13:315–34. https://doi.org/10.1007/s13167-022-00277-2.
Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. Nucl. 2022;65:399–411.
Costa D, Santos R, Da Silva AE, Themistocles BL, da Silva BG, Fialho E, et al. Green tea extract (Camellia sinensis) as a potential antitumoral agent on breast cancer cells (FS13–04–19). Curr Dev Nutr. 2019. https://doi.org/10.1093/cdn/nzz030.FS13-04-19.
Taleb Agha M, Baharetha HM, Al Mansoub MA, Tabana YM, Kaz Abdul Aziz NH, Yam MF, et al. Proapoptotic and antiangiogenic activities of Arctium Lappa L. on breast cancer cell lines. Scientifica (Cairo). 2020. https://doi.org/10.1155/2020/7286053.
Bassiri-Jahromi S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol Rev. 2018. https://doi.org/10.4081/oncol.2018.345.
Ombredane AS, Silva VRP, Andrade LR, Pinheiro WO, Simonelly M, Oliveira JV, et al. In vivo efficacy and toxicity of curcumin nanoparticles in breast cancer treatment: a systematic review. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.612903/full.
Keiler AM, Macejova D, Dietz BM, Bolton JL, Pauli GF, Chen S-N, et al. Evaluation of estrogenic potency of a standardized hops extract on mammary gland biology and on MNU-induced mammary tumor growth in rats. J Steroid Biochem Mol Biol. 2017;174:234–41.
Mushtaq S, Abbasi BH, Uzair B, Abbasi R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018;17:420–51.
Assi S, Torrington E, Cheema E, Al HA. Adverse drug reactions associated with chemotherapeutic agents used in breast cancer: Analysis of patients’ online forums. J Oncol Pharm Pract. 2021;27:108–18. https://doi.org/10.1177/1078155220915767.
Chatterjee P, Gupta S, Banerjee S. Understanding the role of the natural warriors: phytochemicals in breast cancer chemoprevention. Recent Front Phytochem. 2023. https://doi.org/10.1016/B978-0-443-19143-5.00004-9.
Younas M, Hano C, Giglioli-Guivarc’h N, Abbasi BH. Mechanistic evaluation of phytochemicals in breast cancer remedy: current understanding and future perspectives. RSC Adv. 2018;8:29714–44.
Seca A, Pinto D. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci. 2018;19:263.
Sun L, Zhou W, Zhang H, Guo Q, Yang W, Li B, et al. Modulation of multiple signaling pathways of the plant-derived natural products in cancer. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.01153/full.
Sharma E, Attri DC, Sati P, Dhyani P, Szopa A, Sharifi-Rad J, et al. Recent updates on anticancer mechanisms of polyphenols. Front. Cell Dev. Biol. 2022.
Rampogu S, Balasubramaniyam T, Lee J-H. Phytotherapeutic applications of alkaloids in treating breast cancer. Biomed Pharmacother. 2022;155: 113760.
Siraj MA, Islam MA, Al Fahad MA, Kheya HR, Xiao J, Simal-Gandara J. Cancer chemopreventive role of dietary terpenoids by modulating Keap1-Nrf2-ARE signaling system—a comprehensive update. Appl Sci. 2021;11:10806.
Harjotaruno S, Widyawaruyantil A, Sismindari ZNC. Apoptosis inducing effect of andrographolide on TD-47 human breast cancer cell line. African J Tradit Complement Altern Med. 2007. https://doi.org/10.4314/ajtcam.v4i3.31228.
Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu Z, et al. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J Exp Clin Cancer Res. 2018. https://doi.org/10.1186/s13046-018-0926-9.
Xu T, Jiang Y, Yuan S, Zhang L, Chen X, Zhao W, et al. Andrographolide inhibits ER-positive breast cancer growth and enhances fulvestrant efficacy via ROS-FOXM1-ER-α Axis. Front Oncol. 2022;12:1–11.
Lewinska A, Adamczyk-Grochala J, Kwasniewicz E, Deregowska A, Wnuk M. Ursolic acid-mediated changes in glycolytic pathway promote cytotoxic autophagy and apoptosis in phenotypically different breast cancer cells. Apoptosis. 2017. https://doi.org/10.1007/s10495-017-1353-7.
Wang S, Chang X, Zhang J, Li J, Wang N, Yang B, et al. Ursolic acid inhibits breast cancer metastasis by suppressing glycolytic metabolism via activating SP1/Caveolin-1 signaling. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.745584/full.
Wang Z, Zhang P, Jiang H, Sun B, Luo H, Jia A. Ursolic acid enhances the sensitivity of MCF-7 and MDA-MB-231 cells to epirubicin by modulating the autophagy pathway. Molecules. 2022. https://doi.org/10.1371/journal.pone.0000693.
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T Leukemia cells. PLoS ONE. 2007. https://doi.org/10.1371/journal.pone.0000693.
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, et al. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem. 2011;286:6587–601.
Fröhlich T, Mai C, Bogautdinov RP, Morozkina SN, Shavva AG, Friedrich O, et al. Synthesis of tamoxifen-artemisinin and estrogen-artemisinin hybrids highly potent against breast and prostate cancer. ChemMedChem. 2020;15:1473–9. https://doi.org/10.1002/cmdc.202000174.
Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The effect of dual bioactive compounds artemisinin and metformin Co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022. https://doi.org/10.1007/s12010-022-04000-9.
Kim E-S, Jeong C-S, Moon A. Genipin, a constituent of Gardenia jasminoides Ellis, induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep. 2012;27:567–72.
Cho YS. Genipin, an inhibitor of UCP2 as a promising new anticancer agent: a review of the literature. Int J Mol Sci. 2022;23:5637.
Wang Y, Liu X, Wang X, Li F, Li J, Cao W. Furanodiene blocks NF-kB-dependent MMP-9 and VEGF activation and inhibits cellular invasiveness and angiogenesis of breast cancer cells in vitro and in vivo. Biomed Res. 2012;23:231–7.
Zhong Z, Dang Y, Yuan X, Guo W, Li Y, Tan W, et al. Furanodiene, a natural product, inhibits breast cancer growth both in vitro and in vivo. Cell Physiol Biochem. 2012;30:778–90.
Zhong Z, Tan W, Chen X, Wang Y. Furanodiene, a natural small molecule suppresses metastatic breast cancer cell migration and invasion in vitro. Eur J Pharmacol. 2014;737:1–10.
Zhu X-Y, Guo D-W, Lao Q-C, Xu Y-Q, Meng Z-K, Xia B, et al. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci Rep. 2019;9:4541.
Shanmugam MK, Ahn KS, Hsu A, Woo CC, Yuan Y, Tan KHB, et al. Thymoquinone inhibits bone metastasis of breast cancer cells through abrogation of the CXCR4 signaling axis. Front Pharmacol. 2018;9:1294.
El-Far AH, Saddiq AA, Mohamed SA, Almaghrabi OA, Mousa SA. Curcumin and thymoquinone combination attenuates breast cancer cell lines’ progression. Integr Cancer Ther. 2022;21:15347354221099536.
Roy A, Manikkam R. Cytotoxic impact of costunolide isolated from costus speciosus on breast cancer via differential regulation of cell cycle-an in-vitro and in-silico approach. Phytother Res. 2015;29:1532–9.
Choi YK, Seo HS, Choi HS, Choi HS, Kim SR, Shin YC, et al. Induction of Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest and ROS generation by costunolide in estrogen receptor-negative breast cancer cells, MDA-MB-231. Mol Cell Biochem. 2012;363:119–28.
Kim DY, Choi BY. Costunolide—A bioactive sesquiterpene lactone with diverse therapeutic potential. Int J Mol Sci. 2019;20:2926.
Lin S-C, Chu P-Y, Liao W-T, Wu M-Y, Tsui K-H, Lin L-T, et al. Glycyrrhizic acid induces human MDA-MB-231 breast cancer cell death and autophagy via the ROS-mitochondrial pathway. Oncol Rep. 2017;39:703–10.
Azzahra SNA, Hanif N, Hermawan A. MDM2 is a potential target gene of glycyrrhizic acid for circumventing breast cancer resistance to tamoxifen: integrative bioinformatics analysis. Asian Pac J Cancer Prev. 2022;23:2341–50.
Chimplee S, Graidist P, Srisawat T, Sukrong S, Bissanum R, Kanokwiroon K. Anti-breast cancer potential of frullanolide from Grangea maderaspatana plant by inducing apoptosis. Oncol Lett. 2019;17:5283–91.
Heinrich M, Mah J, Amirkia V. Alkaloids used as medicines: structural phytochemistry meets biodiversity—an update and forward look. Molecules. 2021;26:1836.
Chang HC, Chen ST, Chien SY, Kuo SJ, Tsai HT, Chen DR. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum Exp Toxicol. 2011;30:1657–65.
Wu D, Jia H, Zhang Z, Li S. Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol Med Rep. 2020;22:4868–76.
Chen M, Xiao C, Jiang W, Yang W, Qin Q, Tan Q, et al. Capsaicin Inhibits Proliferation and Induces Apoptosis in Breast Cancer by Down-Regulating FBI-1-Mediated NF-κB Pathway. Drug Des Devel Ther. 2021;15:125–40. Available from: https://www.dovepress.com/capsaicin-inhibits-proliferation-and-induces-apoptosis-in-breast-cance-peer-reviewed-article-DDDT
Han B, Wang K, Tu Y, Tan L, He C. Low-dose berberine attenuates the anti-breast cancer activity of chemotherapeutic agents via induction of autophagy and antioxidation. Dose-Response. 2020. https://doi.org/10.1177/1559325820939751.
Pan X, Song Z, Cui Y, Qi M, Wu G, Wang M. Enhancement of sensitivity to tamoxifen by berberine in breast cancer cells by inhibiting ER-α36 expression. Iran J Pharm Res. 2022;21: e126919.
Sun Y, Zhou QQ, Chen F, Gao X, Yang L, Jin X, et al. Berberine inhibits breast carcinoma proliferation and metastasis under hypoxic microenvironment involving gut microbiota and endogenous metabolites. Pharmacol Res. 2023;193: 106817. https://doi.org/10.1016/j.phrs.2023.106817.
Greenshields AL, Doucette CD, Sutton KM, Madera L, Annan H, Yaffe PB, et al. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015;357:129–40.
Buranrat B, Junking M. Piperine suppresses growth and migration of human breast cancer cells through attenuation of Rac1 expression. Asian Pac J Trop Biomed. 2022;12:39.
Jiang F, Ren S, Chen Y, Zhang A, Zhu Y, Zhang Z, et al. Fangchinoline exerts antitumour activity by suppressing the EGFR-PI3K/AKT signalling pathway in colon adenocarcinoma. Oncol Rep. 2021. https://doi.org/10.3892/or.2020.7857.
Xing Z, Zhang Y, Zhang X, Yang Y, Ma Y, Pang D. Fangchinoline induces G1 arrest in breast cancer cells through cell-cycle regulation. Phytother Res. 2013;27:1790–4.
Wang C-D, Yuan C-F, Bu Y-Q, Wu X-M, Wan J-Y, Zhang L, et al. Fangchinoline inhibits cell proliferation via Akt/GSK-3beta/ cyclin D1 signaling and induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pac J Cancer Prev. 2014;15:769–73.
Wang B, Xing Z, Wang F, Yuan X, Zhang Y. Fangchinoline inhibits migration and causes apoptosis of human breast cancer MDA-MB-231 cells. Oncol Lett. 2017;14:5307–12.
Ke Y, Ye K, Grossniklaus HE, Archer DR, Joshi HC, Kapp JA. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother. 2000;49:217–25.
Quisbert-Valenzuela EO, Calaf GM. Apoptotic effect of noscapine in breast cancer cell lines. Int J Oncol. 2016;48:2666–74.
Chougule MB, Patel AR, Jackson T, Singh M. Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PLoS ONE. 2011;6: e17733.
Esnaashari SS, Muhammadnejad S, Amanpour S, Amani A. A combinational approach towards treatment of breast cancer: an analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21:166.
Lu L, Huang R, Wu Y, Jin J-M, Chen H-Z, Zhang L-J, et al. Brucine: a review of phytochemistry, pharmacology, and toxicology. Front Pharmacol. 2020;11:377.
Li M, Li P, Zhang M, Ma F. Brucine suppresses breast cancer metastasis via inhibiting epithelial mesenchymal transition and matrix metalloproteinases expressions. Chin J Integr Med. 2018;24:40–6.
Xu M-R, Wei P-F, Suo M-Z, Hu Y, Ding W, Su L, et al. Brucine suppresses vasculogenic mimicry in human triple-negative breast cancer cell line MDA-MB-231. Biomed Res Int. 2019;2019:6543230.
Grabarska A, Wróblewska-Łuczka P, Kukula-Koch W, Łuszczki JJ, Kalpoutzakis E, Adamczuk G, et al. Palmatine, a bioactive protoberberine alkaloid isolated from berberis cretica, inhibits the growth of human estrogen receptor-positive breast cancer cells and acts synergistically and additively with doxorubicin. Molecules. 2021;26:6253.
Elamin MH, Elmahi AB, Daghestani MH, Al-Olayan EM, Al-Ajmi RA, Alkhuriji AF, et al. Synergistic anti-breast-cancer effects of combined treatment with oleuropein and doxorubicin in vivo. Altern Ther Health Med. 2019;25:17–24.
Ativui S, Danquah CA, Osafo N, Adu W, Ofori M. Palmatine sensitizes chemoresistant triple negative breast cancer cells via efflux inhibition of multidrug resistant protein 1. Sci African. 2021;14: e01022.
Li ZH, Gao J, Hu PH, Xiong JP. Anticancer effects of liriodenine on the cell growth and apoptosis of human breast cancer MCF-7 cells through the upregulation of p53 expression. Oncol Lett. 2017;14:1979–84.
Tran LTT, Dang NYT, Le Nguyen NT, Nguyen HT, Ho DV, Do TT, et al. In silico and in vitro evaluation of alkaloids from Goniothalamus elegans Ast. for breast cancer treatment. Nat Prod Commun. 2022. https://doi.org/10.1177/1934578X221088110.
Das M, Kandimalla R, Gogoi B, Dutta KN, Choudhury P, Devi R, et al. Mahanine, A dietary phytochemical, represses mammary tumor burden in rat and inhibits subtype regardless breast cancer progression through suppressing self-renewal of breast cancer stem cells. Pharmacol Res. 2019;146: 104330.
Satyavarapu EM, Sinha PK, Mandal C. Preclinical development of mahanine-enriched fraction from indian spice murraya koenigii for the management of cancer: efficacy, temperature/pH stability, pharmacokinetics, acute and chronic toxicity (14–180 Days) studies. Biomed Res Int. 2020;2020:1–18.
Ghauri MA, Su Q, Ullah A, Wang J, Sarwar A, Wu Q, et al. Sanguinarine impedes metastasis and causes inversion of epithelial to mesenchymal transition in breast cancer. Phytomedicine. 2021;84: 153500.
Messeha SS, Zarmouh NO, Antonie L, Soliman KFA. Sanguinarine inhibition of TNF-α-induced CCL2, IKBKE/NF-κB/ERK1/2 signaling pathway, and cell migration in human triple-negative breast cancer cells. Int J Mol Sci. 2022;23:8329.
Ren B, Kwah MX-Y, Liu C, Ma Z, Shanmugam MK, Ding L, et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021;515:63–72.
Andreani C, Bartolacci C, Wijnant K, Crinelli R, Bianchi M, Magnani M, et al. Resveratrol fuels HER2 and ERα-positive breast cancer behaving as proteasome inhibitor. Aging (Albany NY). 2017;9:508–23.
Liang Z-J, Wan Y, Zhu D-D, Wang M-X, Jiang H-M, Huang D-L, et al. Resveratrol mediates the apoptosis of triple negative breast cancer cells by reducing POLD1 expression. Front Oncol. 2021;11:1–15.
Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, et al. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS ONE. 2014;9:e102535.
Montalesi E, Cracco P, Acconcia F, Fiocchetti M, Iucci G, Battocchio C, et al. Resveratrol analogs and prodrugs differently affect the survival of breast cancer cells impairing estrogen/estrogen receptor α/neuroglobin pathway. Int J Mol Sci. 2023;24:2148.
Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered. 2019;10:374.
Al-Shaeli SJ, Ethaeb AM, Brown JE. Anti-neoplastic effect of epigallocatechin gallate on breast cancer cells through glucose metabolism. J Phys Conf Ser. 2019;1234: 012073.
Banerjee S, Mandal AKA. Role of epigallocatechin-3- gallate in the regulation of known and novel microRNAs in breast carcinoma cells. Front Genet. 2022;13: 995046.
Cai J, Sun H, Zheng B, Xie M, Xu C, Zhang G, et al. Curcumin attenuates lncRNA H19-induced epithelial-mesenchymal transition in tamoxifen-resistant breast cancer cells. Mol Med Rep. 2021. https://doi.org/10.3892/mmr.2020.11651.
Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, et al. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phyther Res. 2014;28:1553–60.
Coker-Gurkan A, Celik M, Ugur M, Arisan ED, Obakan-Yerlikaya P, Durdu ZB, et al. Curcumin inhibits autocrine growth hormone-mediated invasion and metastasis by targeting NF-κB signaling and polyamine metabolism in breast cancer cells. Amino Acids Springer Vienna. 2018;50:1045–69. https://doi.org/10.1007/s00726-018-2581-z.
Ijaz S, Iqbal J, Abbasi BA, Ullah Z, Yaseen T, Kanwal S, et al. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed Pharmacother. 2023. https://doi.org/10.1016/j.biopha.2023.114687.
Messeha SS, Zarmouh NO, Asiri A, Soliman KFA. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur J Pharmacol. 2020;885: 173419.
Mahmoud MA, Okda TM, Omran GA, Abd-Alhaseeb MM. Rosmarinic acid suppresses inflammation, angiogenesis, and improves paclitaxel induced apoptosis in a breast cancer model via NF3 κB-p53-caspase-3 pathways modulation. J Appl Biomed. 2021;19:202–9.
Sp N, Kang DY, Lee J-M, Bae SW, Jang K-J. Potential antitumor effects of 6-gingerol in p53-dependent mitochondrial apoptosis and inhibition of tumor sphere formation in breast cancer cells. Int J Mol Sci. 2021;22:4660.
Ediriweera MK, Moon JY, Nguyen YT-K, Cho SK. 10-Gingerol Targets Lipid Rafts Associated PI3K/Akt Signaling in Radio-Resistant Triple Negative Breast Cancer Cells. Molecules. 2020;25:3164.
Wala K, Szlasa W, Sauer N, Kasperkiewicz-Wasilewska P, Szewczyk A, Saczko J, et al. Anticancer efficacy of 6-gingerol with paclitaxel against wild type of human breast adenocarcinoma. Molecules. 2022;27:2693.
Tu S-H, Chiou Y-S, Kalyanam N, Ho C-T, Chen L-C, Pan M-H. Garcinol sensitizes breast cancer cells to Taxol through the suppression of caspase-3/iPLA 2 and NF-κB/Twist1 signaling pathways in a mouse 4T1 breast tumor model. Food Funct. 2017;8:1067–79.
Choudhury B, Kandimalla R, Elancheran R, Bharali R, Kotoky J. Garcinia morella fruit, a promising source of antioxidant and anti-inflammatory agents induces breast cancer cell death via triggering apoptotic pathway. Biomed Pharmacother. 2018;103:562–73.
Sudhakaran M, Sardesai S, Doseff AI. Flavonoids: new frontier for immuno-regulation and breast cancer control. Antioxidants. 2019;8:103.
Tuli HS, Garg VK, Bhushan S, Uttam V, Sharma U, Jain A, et al. Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Transl Oncol. 2023;27: 101596.
Cook MT. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer Targets Ther. 2018;10:89–100. Available from: https://www.dovepress.com/mechanism-of-metastasis-suppression-by-luteolin-in-breast-cancer-peer-reviewed-article-BCTT
Lin D, Kuang G, Wan J, Zhang X, Li H, Gong X, et al. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol Rep. 2017;37:895–902.
Tsai K-J, Tsai H-Y, Tsai C-C, Chen T-Y, Hsieh T-H, Chen C-L, et al. Luteolin inhibits breast cancer stemness and enhances chemosensitivity through the Nrf2-mediated pathway. Molecules. 2021;26:6452.
Noori S, Tavirani MR, Deravi N, Rabbani MIM, Zarghi A. Naringenin enhances the anti-cancer effect of cyclophosphamide against mda-mb-231 breast cancer cells via targeting the stat3 signaling pathway. Iran J Pharm Res. 2020;19:122–33.
Zhao Z, Jin G, Ge Y, Guo Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology. 2019;27:1021–36. https://doi.org/10.1007/s10787-018-00556-3.
Basta AH, Lotfy VF, Ghaly NS, Nabil M, Mohamed KM. Bioactivity evaluation of amino acid-conjugates with protein versus cellulose based conjugates and extracted flavonoids. J Drug Deliv Sci Technol. 2020;60: 101924.
Atta EM, Hegab KH, Abdelgawad AAM, Youssef AA. Synthesis, characterization and cytotoxic activity of naturally isolated naringin-metal complexes. Saudi Pharm J. 2019;27:584–92.
Yang D, Guo Q, Liang Y, Zhao Y, Tian X, Ye Y, et al. Wogonin induces cellular senescence in breast cancer via suppressing TXNRD2 expression. Arch Toxicol. 2020;94:3433–47.
Maryam R. Wogonin from scutellaria baicalensis-induced radioresistance in MCF-7 breast cancer cell line. Eurasian J Med Oncol. 2022. https://doi.org/10.14744/ejmo.2022.17217.
Jiang H, Fan J, Cheng L, Hu P, Liu R. The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. Onco Targets Ther. 2018;11:8153–63.
Zhao Q, Zhao M, Parris AB, Xing Y, Yang X. Genistein targets the cancerous inhibitor of PP2A to induce growth inhibition and apoptosis in breast cancer cells. Int J Oncol. 2016;49:1203–10.
Shukla RP, Dewangan J, Urandur S, Banala VT, Diwedi M, Sharma S, et al. Multifunctional hybrid nanoconstructs facilitate intracellular localization of doxorubicin and genistein to enhance apoptotic and anti-angiogenic efficacy in breast adenocarcinoma. Biomater Sci. 2020;8:1298–315.
Kim GY, Suh J, Jang J-H, Kim D-H, Park OJ, Park SK, et al. Genistein Inhibits Proliferation of BRCA1 Mutated Breast Cancer Cells: The GPR30-Akt Axis as a Potential Target. J Cancer Prev. 2019;24:197–207.
Binienda A, Ziolkowska S, Pluciennik E. The anticancer properties of silibinin: its molecular mechanism and therapeutic effect in breast cancer. Anticancer Agents Med Chem. 2020;20:1787–96.
Molavi O, Narimani F, Asiaee F, Sharifi S, Tarhriz V, Shayanfar A, et al. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm Biol. 2017;55:729–39.
Maasomi ZJ, Soltanahmadi YP, Dadashpour M, Alipour S, Abolhasani S, Zarghami N. Synergistic anticancer effects of silibinin and chrysin in T47D breast cancer cells. Asian Pacific J Cancer Prev. 2017;18:1283.
Nawaz Q, Fuentes-Chandía M, Tharmalingam V, Ur Rehman MA, Leal-Egaña A, Boccaccini AR. Silibinin releasing mesoporous bioactive glass nanoparticles with potential for breast cancer therapy. Ceram Int. 2020. https://doi.org/10.2139/ssrn.3473993.
Iqbal MA, Chattopadhyay S, Siddiqui FA, Ur Rehman A, Siddiqui S, Prakasam G, et al. Silibinin induces metabolic crisis in triple-negative breast cancer cells by modulating EGFR-MYC-TXNIP axis: potential therapeutic implications. FEBS J. 2021;288:471–85.
Datta S, Saha P, Dey S, Sinha D. Natural Products as Chemosensitizers for Adjunct Therapy in Cancer Management. In: Kumar M, Sharma A, Kumar P, editors. Pharmacother Bot Cancer Chemoprevention. Singapore: Springer Singapore; 2020. p. 67–119.
Sharma P, Khan MA, Najmi AK, Chaturvedi S, Akhtar M. Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Med Oncol. 2022;39:248.
Han S-H, Lee J-H, Woo J-S, Jung G-H, Jung S-H, Han E-J, et al. Myricetin induces apoptosis through the MAPK pathway and regulates JNK-mediated autophagy in SK-BR-3 cells. Int J Mol Med. 2022;49:54.
Sajedi N, Homayoun M, Mohammadi F, Soleimani M. Myricetin exerts its apoptotic effects on MCF-7 breast cancer cells through evoking the BRCA1-GADD45 pathway. Asian Pac J Cancer Prev. 2020. https://doi.org/10.31557/APJCP.2020.21.12.3461.
Mitra S, Dash R. Natural products for the management and prevention of breast cancer. Evidence-Based Complement Altern Med. 2018;2018:1–23.
Scaria B, Sood S, Raad C, Khanafer J, Jayachandiran R, Pupulin A, et al. Natural health products (NHP’s) and natural compounds as therapeutic agents for the treatment of cancer; mechanisms of anti-cancer activity of natural compounds and overall trends. Int J Mol Sci. 2020;21:1–32.
More MP, Pardeshi SR, Pardeshi CV, Sonawane GA, Shinde MN, Deshmukh PK, et al. Recent advances in phytochemical-based Nano-formulation for drug-resistant Cancer. Med Drug Discov. 2021;10: 100082.
Koh Y-C, Ho C-T, Pan M-H. Recent advances in cancer chemoprevention with phytochemicals. J food drug Anal. 2020;28:14–37.
Acknowledgements
Authors are thankful to the Department of Science and Technology (DST), Government of India for providing Departmental DST-FIST grant (SR/FST/LSI-656/2016) to the Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, India.
Author information
Authors and Affiliations
Contributions
P.S. conceptualized and supervised the overall studies, critically reviewed, and wrote the final draft of the manuscript. K.G. performed data curing. SKK. performed data curing. SM. contributed toward review and editing. S.T. provided broad direction for the study, supervised, critically reviewed, and finally approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no ethical, legal, and financial conflicts related to the article.
Consent for publication
All authors read and approved the manuscript to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, P., Gupta, K., Khandai, S.K. et al. Phytometabolites as modulators of breast cancer: a comprehensive review of mechanistic insights. Med Oncol 41, 45 (2024). https://doi.org/10.1007/s12032-023-02269-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02269-2