Abstract
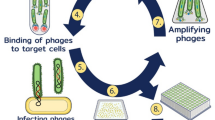
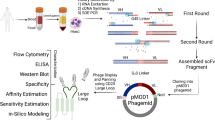
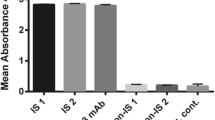
Lymphocyte function-associated antigene-1 (LFA-1) is a well-described integrin found on lymphocytes and other leukocytes, which is known to be overexpressed in leukemias and lymphomas. This receptor plays a significant role in immune responses such as T-cell activation, leukocyte cell–cell interactions, and trafficking of leukocyte populations. Subsequently, binders of LFA-1 emerge as potential candidates for cancer and autoimmune therapy. This study used the phage display technique to construct and characterize a high-affinity single-chain fragment variable (scFv) antibody against LFA-1. After expression, purification, dialysis, and concentration of the recombinant LFA-1 protein, four female BALB/c mice were immunized, splenocyte’s mRNA was extracted, and cDNA was synthesized. A scFv library was constructed by linking the amplified VH/Vκ fragments through a 72-bp linker using SOEing PCR. Next, the scFv gene fragments were cloned into the pComb-3XSS phagemid vector; thus, the phage library was developed. The selection process involved three rounds of phage-bio-panning, polyclonal, and monoclonal phage ELISA. AF17 was chosen and characterized among the positive clones through SDS-PAGE, Western blotting, indirect ELISA, and in-silico analyses. The results of the study showed the successful construction of a high-affinity scFv library against LFA-1. The accuracy of the AF17 production and its ability to bind to the LFA-1 were confirmed through SDS-PAGE, Western blot, and ELISA. This study highlights the potential application of the high-affinity AF17 against LFA-1 for targeting T lymphocytes for therapeutic purposes.








Similar content being viewed by others
Data availability
Certain data are confidential but might be available from the corresponding author upon reasonable request.
References
Li D, Molldrem JJ, Ma Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J Biol Chem. 2009;284(31):21001–10.
Pflugfelder SC, Stern M, Zhang S, Shojaei A. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease. J Ocul Pharmacol Ther. 2017;33(1):5–12.
Kozani PS, Shabani S. Adverse events and side effects of chimeric antigen receptor (CAR) T cell therapy in patients with hematologic malignancies. Trends Med Sci. 2021;1(1):e116301.
Reina M, Espel E. Role of LFA-1 and ICAM-1 in cancer. Cancers. 2017;9(11):153.
Phongpradist R, Chittasupho C, Okonogi S, Siahaan T, Anuchapreeda S, Ampasavate C, Berkland C. LFA-1 on leukemic cells as a target for therapy or drug delivery. Curr Pharm Des. 2010;16(21):2321–30.
Hust M, Meyer T, Voedisch B, Rülker T, Thie H, El-Ghezal A, Kirsch MI, Schütte M, Helmsing S, Meier D, Schirrmann T. A human scFv antibody generation pipeline for proteome research. J Biotechnol. 2011;152(4):159–70.
Kumar R, Parray HA, Shrivastava T, Sinha S, Luthra K. Phage display antibody libraries: a robust approach for generation of recombinant human monoclonal antibodies. Int J Biol Macromol. 2019;135:907–18.
Kaboli PJ, Shabani S, Sharma S, Nasr MP, Yamaguchi H, Hung MC. Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates. Am J Cancer Res. 2022;12(4):1671.
Nikolova G, Georgieva Y, Atanasova A, Radulova G, Kapogianni A, Tsacheva I. Autoinduction as means for optimization of the heterologous expression of recombinant single-chain Fv (scFv) antibodies. Mol Biotechnol. 2021;63(11):1049–56.
Accardi L, Di Bonito P. Antibodies in single-chain format against tumour-associated antigens: present and future applications. Curr Med Chem. 2010;17(17):1730–55.
Kuhn P, Fühner V, Unkauf T, Moreira GM, Frenzel A, Miethe S, Hust M. Recombinant antibodies for diagnostics and therapy against pathogens and toxins generated by phage display. Proteom Clin Appl. 2016;10(9–10):922–48.
Jeong KJ, Jang SH, Velmurugan N. Recombinant antibodies: engineering and production in yeast and bacterial hosts. Biotechnol J. 2011;6(1):16–27.
Miethe S, Meyer T, Wöhl-Bruhn S, Frenzel A, Schirrmann T, Dübel S, Hust M. Production of single chain fragment variable (scFv) antibodies in Escherichia coli using the LEX™ bioreactor. J Biotechnol. 2013;163(2):105–11.
Shukra AM, Sridevi NV, Chandran D, Maithal K. Production of recombinant antibodies using bacteriophages. Eur J Microbiol Immunol. 2014;4(2):91–8.
Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M. scFv antibody: principles and clinical application. J Immunol Res. 2012;2012.
Fath MK, Naderi M, Hamzavi H, Ganji M, Shabani S, Khalesi B, Pourzardosht N, Hashemi ZS, Khalili S. Molecular Mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J Trace Elem Med Biol. 2022;73:127044.
André AS, Moutinho I, Dias JN, Aires-da-Silva F. In vivo Phage Display: a promising selection strategy for the improvement of antibody targeting and drug delivery properties. Front Microbiol. 2022;13:962124.
Shabani S, Moghadam MF, Gargari SL. Isolation and characterization of a novel GRP78-specific single-chain variable fragment (scFv) using ribosome display method. Med Oncol. 2021;38(9):115.
Ledsgaard L, Kilstrup M, Karatt-Vellatt A, McCafferty J, Laustsen AH. Basics of antibody phage display technology. Toxins. 2018;10(6):236.
Aghebati-Maleki L, Bakhshinejad B, Baradaran B, Motallebnezhad M, Aghebati-Maleki A, Nickho H, Yousefi M, Majidi J. Phage display as a promising approach for vaccine development. J Biomed Sci. 2016;23:1–8.
Sioud M. Phage display libraries: from binders to targeted drug delivery and human therapeutics. Mol Biotechnol. 2019;61(4):286–303.
Yu H, Yang Z, Li F, Xu L, Sun Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020;27(1):1425–37.
Hashemi A, Bigdeli R, Shahnazari M, Oruji F, Fattahi S, Panahnejad E, Ghadri A, Movahedi-Asl E, Mahdavi-Ourtakand M, Asgary V, Baghbani-Arani F. Evaluation of inflammasome activation in peripheral blood mononuclear cells of hemodialysis treated patients with glomerulonephritis. Iran J Pharm Res. 2021;20(3):609.
Oruji F, Baghbani Arani F, Mahdavi OM. Evaluation of the gene expression of IL-1β and Casp-1 related to inflammation process in glomerulonephritis patients. J Anim Environ. 2018;10(3):477–82.
Ohashi Y, Minegishi M, Tsuchiya S, Konno T. Three monoclonal antibodies against human LFA-1 α and β chains with different biological activities. Tohoku J Exp Med. 1992;168(4):599–610.
Leonardi CL. Efalizumab: an overview. J Am Acad Dermatol. 2003;49(2):98–104.
Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10(5):345–52.
Gaughan CL. The present state of the art in expression, production and characterization of monoclonal antibodies. Mol Divers. 2016;20(1):255–70.
Mehralizadeh H, Nazari A, Oruji F, Roostaie M, Hosseininozari G, Yazdani O, Esbati R, Roudini K. Cytokine sustained delivery for cancer therapy; special focus on stem cell-and biomaterial-based delivery methods. Pathol Res Pract. 2023;9:154528.
Reader RH, Workman RG, Maddison BC, Gough KC. Advances in the production and batch reformatting of phage antibody libraries. Mol Biotechnol. 2019;61:801–15.
Bashir S, Paeshuyse J. Construction of antibody phage libraries and their application in veterinary immunovirology. Antibodies. 2020;9(2):21.
Kaplon H, Chenoweth A, Crescioli S, Reichert JM. Antibodies to watch in 2022. In: MAbs 2022 (Vol. 14, No. 1, p. 2014296).
Anand T, Virmani N, Bera BC, Vaid RK, Vashisth M, Bardajatya P, Kumar A, Tripathi BN. Phage display technique as a tool for diagnosis and antibody selection for coronaviruses. Curr Microbiol. 2021;78(4):1124–34.
Dong Y, Meng F, Wang Z, Yu T, Chen A, Xu S, Wang J, Yin M, Tang L, Hu C, Wang H. Construction and application of a human scFv phage display library based on Cre-LoxP recombination for anti-PCSK9 antibody selection. Int J Mol Med. 2021;47(2):708–18.
Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindqvist E, Schier R, Hemingsen G, Wong C, Gerhart JC, Marks JD. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci. 1998;95(11):6157–62.
Pardon E, Laeremans T, Triest S, Rasmussen SG, Wohlkönig A, Ruf A, Muyldermans S, Hol WG, Kobilka BK, Steyaert J. A general protocol for the generation of Nanobodies for structural biology. Nat Protoc. 2014;9(3):674–93.
Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987;100(1–2):173–9.
Acknowledgements
This paper has been extracted from a M.Sc. thesis. The authors would like to acknowledge Tarbiat Modares University for funding this project. I also wish to thank Dr. Faezeh Noorabad ghahroodi as well as Miss. Shima Shabani for their kind consulting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Fatemeh Afsharnoori and Mehdi Forouzandeh-Moghadam declare that they have no conflict of interest.
Ethical approval
All the experiments reported herein have been performed completely in accordance with standard animal welfare regulations as approved by Tarbiat Modares University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afsharnoori, F., Forouzandeh Moghadam, M. Isolation and characterization of a novel single-chain variable fragment (scFv) against Lymphocyte function-associated antigen-1 (LFA-1) using phage display method. Med Oncol 41, 15 (2024). https://doi.org/10.1007/s12032-023-02242-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02242-z