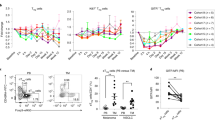
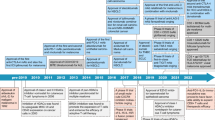
Abstract
Tumour necrosis factor receptor 2 or TNFR2 is considered an appealing target protein due to its limited frequency to TREGs, which are highly immunosuppressive and present on human malignancies. Numerous studies have revealed that TNFR2 is primarily found on MDSCs (myeloid-derived suppressor cells) and CD + Foxp3 + regulatory T cells (TREGs). Therefore, it has great importance in the proliferation and functional activity of TREGs and MDSCs. TNFR2 suppression must be downregulated or upregulated as required to treat malignancies and diseases like autoimmune disorders. Therefore, at the molecular level, advances in the comprehension of TNFR2's complex structure and its binding to TNF have opened the door to structure-guided drug development. Two critical obstacles to cancer treatment are the dearth of TREG-specific inhibitors and the lack of widely applicable ways to target tumours via frequently expressed surface oncogenes directly. Many researchers have discovered potential antagonists and agonists of TNFR2, which were successful in inhibiting TREGs proliferation, reducing soluble TNFR2 secretion from normal cells, and expanding T effector cells. The data represented in the following review article elucidates the clinically administrated TNFR2 antagonist and agonist in treating cancers.


Similar content being viewed by others
Data availability
N/A.
References
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. https://doi.org/10.1056/NEJMoa1504030.
Baeyens KJ, De Bondt HL, Raeymaekers A, Fiers W, De Ranter CJ. The structure of mouse tumour-necrosis factor at A resolution: towards modulation of its selectivity and trimerization. Acta Crystallogr D Biol Crystallogr. 1999;55(Pt 4):772–8. https://doi.org/10.1107/s0907444998018435.
Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J. 2011;278(6):888–98. https://doi.org/10.1111/j.1742-4658.2011.08017.x.
Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol. 2006;169(5):1886–98. https://doi.org/10.2353/ajpath.2006.060603.
Chen X, Subleski JJ, Kopf H, Howard OM, Männel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol (Baltimore, Md). 2008;180(10):6467–71. https://doi.org/10.4049/jimmunol.180.10.6467.
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Investig. 2012;122(11):4094–104. https://doi.org/10.1172/JCI64115.
Böcker W, Docheva D, Prall WC, Egea V, Pappou E, Roßmann O, et al. IKK-2 is required for TNF-α-induced invasion and proliferation of human mesenchymal stem cells. J Mol Med. 2008;86(10):1183–92.
Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. https://doi.org/10.1146/annurev.immunol.25.022106.141623.
Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Grégoire S, Martin GH, Elhage R, Derian N, Carpentier W, Marodon G, Klatzmann D, Piaggio E, Salomon BL. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting TREGs. J Clin Investig. 2010;120(12):4558–68. https://doi.org/10.1172/JCI42945.
Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol (Baltimore, Md). 2013;190(3):1076–84. https://doi.org/10.4049/jimmunol.1202659.
Zaragoza B, Chen X, Oppenheim JJ, Baeyens A, Gregoire S, Chader D, Gorochov G, Miyara M, Salomon BL. Suppressive activity of human regulatory T cells is maintained in the presence of TNF. Nat Med. 2016;22(1):16–7. https://doi.org/10.1038/nm.4019.
Quazi S. Elucidation of CRISPR-Cas9 application in novel cellular immunotherapy. Preprints. 2021. https://doi.org/10.20944/preprints202108.0387.v1).
Chopra M, Biehl M, Steinfatt T, Brandl A, Kums J, Amich J, Vaeth M, Kuen J, Holtappels R, Podlech J, Mottok A, Kraus S, Jordán-Garrote AL, Bäuerlein CA, Brede C, Ribechini E, Fick A, Seher A, Polz J, Ottmüller KJ, et al. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med. 2016;213(9):1881–900. https://doi.org/10.1084/jem.2015156.
Leclerc M, Naserian S, Pilon C, Thiolat A, Martin GH, Pouchy C, Dominique C, Belkacemi Y, Charlotte F, Maury S, Salomon BL, Cohen JL. Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells. Blood. 2016;128(12):1651–9. https://doi.org/10.1182/blood-2016-02-700849.
Pierini A, Strober W, Moffett C, Baker J, Nishikii H, Alvarez M, Pan Y, Schneidawind D, Meyer E, Negrin RS. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. 2016;128(6):866–71. https://doi.org/10.1182/blood-2016-04-711275.
Chen X, Bäumel M, Männel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol (Baltimore, Md). 2007;179(1):154–61. https://doi.org/10.4049/jimmunol.179.1.154.
Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–56.
Heinrich M, Burger D, Wang L, Tahhan G, Reinhold P, Zhao M, et al. TNFR1 and TNFR2 expression and induction on human TREG cells from type 1 diabetic subjects. Antibodies. 2015;4(1):34–47.
Polz J, Remke A, Weber S, Schmidt D, Weber-Steffens D, Pietryga-Krieger A, Müller N, Ritter U, Mostböck S, Männel DN. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immunity Inflamm Disease. 2014;2(2):121–30. https://doi.org/10.1002/iid3.19.
Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Can Res. 2012;72(9):2162–71. https://doi.org/10.1158/0008-5472.CAN-11-3687.
Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Can Res. 2011;71(22):6915–20. https://doi.org/10.1158/0008-5472.CAN-11-1156.
Gavas S, Quazi S, Karpiński T. Nanoparticles for cancer therapy: current progress and challenges. Preprints. 2021. https://doi.org/10.20944/preprints202108.0218.v1.
Teng MW, Ritchie DS, Neeson P, Smyth MJ. Biology and clinical observations of regulatory T cells in cancer immunology. Cancer Immunol Immunother. 2010;344:61–95.
Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40(4):1099–106. https://doi.org/10.1002/eji.200940022.
Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, Plebanski M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ TREGs within the tumor microenvironment. Clin Immunol (Orlando, Fla). 2013;149(1):97–110. https://doi.org/10.1016/j.clim.2013.07.003.
Hamilton KE, Simmons JG, Ding S, Van Landeghem L, Lund PK. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol cancer Res. 2011;9(12):1718–31. https://doi.org/10.1158/1541-7786.MCR-10-0210.
Ungewickell A, Bhaduri A, Rios E, Reuter J, Lee CS, Mah A, Zehnder A, Ohgami R, Kulkarni S, Armstrong R, Weng WK, Gratzinger D, Tavallaee M, Rook A, Snyder M, Kim Y, Khavari PA. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47(9):1056–60. https://doi.org/10.1038/ng.3370.
Nakayama S, Yokote T, Tsuji M, Akioka T, Miyoshi T, Hirata Y, Hiraoka N, Iwaki K, Takayama A, Nishiwaki U, Masuda Y, Hanafusa T. Expression of tumour necrosis factor-α and its receptors in Hodgkin lymphoma. Br J Haematol. 2014;167(4):574–7. https://doi.org/10.1111/bjh.13015.
Wang J, Al-Lamki RS. Tumor necrosis factor receptor 2: its contribution to acute cellular rejection and clear cell renal carcinoma. Biomed Res Int. 2013. https://doi.org/10.1155/2013/821310.
Rauert H, Stühmer T, Bargou R, Wajant H, Siegmund D. TNFR1 and TNFR2 regulate the extrinsic apoptotic pathway in myeloma cells by multiple mechanisms. Cell Death Dis. 2011;2(8):e194. https://doi.org/10.1038/cddis.2011.78.
Uhlén M, Björling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C, Berglund L, Bergström K, Brumer H, Cerjan D, Ekström M, Elobeid A, Eriksson C, Fagerberg L, Falk R, Fall J, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4(12):1920–32. https://doi.org/10.1074/mcp.M500279-MCP200.
Welborn MB 3rd, Van Zee K, Edwards PD, Pruitt JH, Kaibara A, Vauthey JN, Rogy M, Castleman WL, Lowry SF, Kenney JS, Stüber D, Ettlin U, Wipf B, Loetscher H, Copeland EM 3rd, Lesslauer W, Moldawer LL. A human tumor necrosis factor p75 receptor agonist stimulates in vitro T cell proliferation but does not produce inflammation or shock in the baboon. J Exp Med. 1996;184(1):165–71. https://doi.org/10.1084/jem.184.1.165.
Van Zee KJ, Stackpole SA, Montegut WJ, Rogy MA, Calvano SE, Hsu KC, Chao M, Meschter CL, Loetscher H, Stüber D. A human tumor necrosis factor (TNF) alpha mutant that binds exclusively to the p55 TNF receptor produces toxicity in the baboon. J Exp Med. 1994;179(4):1185–91. https://doi.org/10.1084/jem.179.4.1185.
Chopra M, Riedel SS, Biehl M, Krieger S, von Krosigk V, Bäuerlein CA, Brede C, Jordan Garrote AL, Kraus S, Schäfer V, Ritz M, Mattenheimer K, Degla A, Mottok A, Einsele H, Wajant H, Beilhack A. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. 2013;34(6):1296–303. https://doi.org/10.1093/carcin/bgt038.
Sasi SP, Bae S, Song J, Perepletchikov A, Schneider D, Carrozza J, Yan X, Kishore R, Enderling H, Goukassian DA. Therapeutic non-toxic doses of TNF induce significant regression in TNFR2-p75 knockdown Lewis lung carcinoma tumor implants. PLOS ONE. 2014;9:e92373.
Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou W, Gan H, Wang J, Jiang X, Yin B, Li Z. Targeting transmembrane TNF-α suppresses breast cancer growth. Can Res. 2013;73(13):4061–74. https://doi.org/10.1158/0008-5472.CAN-12-3946.
Ham B, Wang N, D’Costa Z, Fernandez MC, Bourdeau F, Auguste P, Illemann M, Eefsen RL, Høyer-Hansen G, Vainer B, Evrard M, Gao ZH, Brodt P. TNF Receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Can Res. 2015;75(24):5235–47. https://doi.org/10.1158/0008-5472.CAN-14-3173.
Quazi S. Artificial intelligence and machine learning in precision and genomic medicine. Preprints. 2021. https://doi.org/10.20944/preprints202110.0011.v1.
Chang LY, Lin YC, Chiang JM, Mahalingam J, Su SH, Huang CT, Chen WT, Huang CH, Jeng WJ, Chen YC, Lin SM, Sheen IS, Lin CY. Blockade of TNF-α signaling benefits cancer therapy by suppressing effector regulatory T cell expansion. Oncoimmunology. 2015;4(10):e1040215. https://doi.org/10.1080/2162402X.2015.1040215.
Jain SS, Bird RP. Elevated expression of tumor necrosis factor-alpha signaling molecules in colonic tumors of Zucker obese (fa/fa) rats. Int J Cancer. 2010;127(9):2042–50. https://doi.org/10.1002/ijc.25232.
Quazi S. An overview of CAR T cell mediated B Cell maturation antigen therapy. Preprints. 2021. https://doi.org/10.20944/preprints202109.0212.v1.
T. J. Labs, B6.129S7-Tnfrsf1b; www.jax.org/strain/003246
Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard OM, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3- conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J Immunol (Baltimore, Md). 2010;185(1):174–82. https://doi.org/10.4049/jimmunol.0903548.
Babic A, Shah SM, Song M, Wu K, Meyerhardt JA, Ogino S, Yuan C, Giovannucci EL, Chan AT, Stampfer MJ, Fuchs CS, Ng K. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br J Cancer. 2016;114(9):995–1002. https://doi.org/10.1038/bjc.2016.85.
Cui LF, Guo XJ, Wei J, Liu FF, Fan Y, Lang RG, Gu F, Zhang XM, Fu L. Overexpression of TNF-alpha and TNFRII in invasive micropapillary carcinoma of the breast: clinicopathological correlations. Histopathology. 2008;53(4):381–8. https://doi.org/10.1111/j.1365-2559.2008.03128.x.
Okubo Y, Mera T, Wang L, Faustman DL. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci Rep. 2013;3:3153. https://doi.org/10.1038/srep03153.
Govindaraj C, Tan P, Walker P, Wei A, Spencer A, Plebanski M. Reducing TNF receptor 2+ regulatory T cells via the combined action of azacitidine and the HDAC inhibitor, panobinostat for clinical benefit in acute myeloid leukemia patients. Clin Cancer Res. 2014;20(3):724–35. https://doi.org/10.1158/1078-0432.CCR-13-1576.
Yan F, Du R, Wei F, Zhao H, Yu J, Wang C, Zhan Z, Ding T, Ren X, Chen X, Li H. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol Immunother. 2015;64(11):1475–85. https://doi.org/10.1007/s00262-015-1751-z.
Nakayama S, Iida K, Tsuzuki T, Iwashita T, Murakami H, Asai N, Iwata Y, Ichihara M, Ito S, Kawai K, Asai M, Kurokawa K, Takahashi M. Implication of expression of GDNF/Ret signalling components in differentiation of bone marrow haemopoietic cells. Br J Haematol. 1999;105(1):50–7.
Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19(3):322–8. https://doi.org/10.1038/nm.3085.
He X, Landman S, Bauland SC, van den Dolder J, Koenen HJ, Joosten I. A TNFR2-agonist facilitates high purity expansion of human low purity TREG cells. PLoS ONE. 2016;11(5):e0156311. https://doi.org/10.1371/journal.pone.0156311.
Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–61. https://doi.org/10.1182/blood-2005-11-4567.
McGovern JL, Nguyen DX, Notley CA, Mauri C, Isenberg DA, Ehrenstein MR. Th17 cells are restrained by TREG cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012;64(10):3129–38. https://doi.org/10.1002/art.34565.
Urbano P, Koenen H, Joosten I, He X. An autocrine TNFα-tumor necrosis factor receptor 2 loop promotes epigenetic effects inducing human TREG stability in vitro. Front Immunol. 2018;9:573. https://doi.org/10.3389/fimmu.2018.00573.
Miller PG, Bonn MB, McKarns SC. Transmembrane TNF-TNFR2 impairs Th17 differentiation by promoting Il2 expression. J Immunol (Baltimore, Md). 2015;195(6):2633–47. https://doi.org/10.4049/jimmunol.1500286.
Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15(6):580–7. https://doi.org/10.1038/ni.2868.
Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. J Exp Med. 2016;213(7):1241–53. https://doi.org/10.1084/jem.20151255.
Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol. 2014;5:46.
Mougiakakos D, Johansson CC, Jitschin R, Böttcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117(3):857–61. https://doi.org/10.1182/blood-2010-09-307041.
Okubo Y, Torrey H, Butterworth J, Zheng H, Faustman DL. TREG activation defect in type 1 diabetes: correction with TNFR2 agonism. Clin Transl Immunol. 2016;5(1):e56. https://doi.org/10.1038/cti.2015.43.
Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, et al. Effective chemoimmunotherapy with anti-TGFβ antibody and cyclophosphamide in a mouse model of breast cancer. PLoS ONE. 2014;9(1):e85398.
Yan F, Du R, Wei F, Zhao H, Yu J, Wang C, Zhan Z, Ding T, Ren X, Chen X, Li H. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol Immunother. 2015;64(11):1475–85. https://doi.org/10.1007/s00262-015-1751-z.
Tirosh I, Izar B, Prakadan SM, Wadsworth MH 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science (New York, NY). 2016;352(6282):189–96. https://doi.org/10.1126/science.aad0501.
Torrey H, Butterworth J, Mera T, Okubo Y, Wang L, Baum D, Defusco A, Plager S, Warden S, Huang D, Vanamee E, Foster R, Faustman DL. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated TREGs. Sci signal. 2017;10(462):eaaf8608. https://doi.org/10.1126/scisignal.aaf8608.
Nie Y, He J, Shirota H, Trivett AL, Yang D, Klinman DM, Oppenheim JJ, Chen X. Blockade of TNFR2 signaling enhances the immunotherapeutic effect of CpG ODN in a mouse model of colon cancer. Sci signal. 2018;11(511):eaan0790. https://doi.org/10.1126/scisignal.aan0790.
Vanamee ÉS, Faustman DL. TNFR2: a novel target for cancer immunotherapy. Trends Mol Med. 2017;23(11):1037–46.
Filbert E, Krishnan S, Alvarado R, Huang G, Bahjat F, Yang X. 693 APX601, a Novel TNFR2 antagonist antibody for cancer immunotherapy. J Immunother Cancer. 2020;8(Suppl 3):A417–A417.
Case K, Tran L, Yang M, Zheng H, Kuhtreiber WM, Faustman DL. TNFR2 blockade alone or in combination with PD-1 blockade shows therapeutic efficacy in murine cancer models. J Leukoc Biol. 2020;107(6):981–91.
Torrey H, Khodadoust M, Tran L, Baum D, Defusco A, Kim YH, Faustman DL. Targeted killing of TNFR2-expressing tumor cells and TREGs by TNFR2 antagonistic antibodies in advanced Sézary syndrome. Leukemia. 2019;33(5):1206–18. https://doi.org/10.1038/s41375-018-0292-9.
Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188(4):1592–9. https://doi.org/10.4049/jimmunol.1101304.
Torrey H, Kühtreiber WM, Okubo Y, Tran L, Case K, Zheng H, Vanamee E, Faustman DL. A novel TNFR2 agonist antibody expands highly potent regulatory T cells. Sci signal. 2020;13(661):eaba9600. https://doi.org/10.1126/scisignal.aba9600.
Zou H, Li R, Hu H, Hu Y, Chen X. Modulation of regulatory T Cell activity by TNF receptor type II-targeting pharmacological agents. Front Immunol. 2018;9:594. https://doi.org/10.3389/fimmu.2018.00594.
Atretkhany KSN, Mufazalov IA, Dunst J, Kuchmiy A, Gogoleva VS, Andruszewski D, et al. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc Natl Acad Sci. 2018;115(51):13051–6.
Faustman DL. TNF, TNF inducers, and TNFR2 agonists: a new path to type 1 diabetes treatment. Diabetes Metab Res Rev. 2018;34(1):e2941.
Quazi S. Role of artificial intelligence and machine learning in bioinformatics: drug discovery and drug repurposing. Preprints. 2021. https://doi.org/10.20944/preprints202105.0346.v1.
Medler J, Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opin Ther Targets. 2019;23(4):295–307.
Tam EM, Fulton RB, Sampson JF, Muda M, Camblin A, Richards J, et al. Antibody-mediated targeting of TNFR2 activates CD8+ T cells in mice and promotes antitumor immunity. Science translational medicine. 2019. https://doi.org/10.1126/scitranslmed.aax0720.
Fulton RB, Camblin A, Sampson JF, Richards J, Wong C, Koshkaryev A, et al. Mechanism of action of a novel agonist TNFR2 antibody that induces co-stimulation of T cells and promotes robust anti-tumor immunity. Cancer Res. 2019;79(13 Supplement):3270.
Sampson JF, Fulton RB, Kurella VB, Richards JM, Camblin AJ, Wong CS, et al. A novel TNFR2 antibody induces T cell co-stimulation and promotes durable anti-tumor immunity. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aax0720.
Richards J, Wong C, Koshkaryev A, Fulton R, Camblin A, Sampson J, et al. MM-401, a novel anti-TNFR2 antibody that induces T cell co-stimulation, robust anti-tumor activity and immune memory. Cancer Res. 2019;79(13 Supplement):4846.
Sampson JF, Kurella VB, Paragas V, Kumar S, Lulo JE, Qiu JA, et al. A novel human TNFR2 antibody (MM-401) modulates T cell responses in anti-cancer immunity. Cancer Res. 2019;79(13 Supplement):555.
Williams GS, Mistry B, Guillard S, Ulrichsen JC, Sandercock AM, Wang J, et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget. 2016;7(42):68278.
Sheehan KC, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV, Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med. 1995;181(2):607–17.
Li F, Ravetch JV. A general requirement for FcγRIIB co-engagement of agonistic anti-TNFR antibodies. Cell Cycle. 2012;11(18):3343–4.
Quazi S, Jangi R. Artificial intelligence and machine learning in medicinal chemistry and validation of emerging drug targets. Preprints. 2021. https://doi.org/10.20944/preprints202105.0567.v1.
Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J Immunother Cancer. 2014;2(1):1–10.
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–72.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. https://doi.org/10.1038/nature13904.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. https://doi.org/10.1056/NEJMoa1810865.
Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016;21(10):1230–40. https://doi.org/10.1634/theoncologist.2016-0055.
Incidence and Clinical Impact of Anti-TNFα Treatment of Severe Immune Checkpoint Inhibitor-induced Colitis in Advanced Melanoma: The Mecolit Survey.
Lesage C, Longvert C, Prey S, Maanaoui S, Dréno B, Machet L, Zehou O, Kramkimel N, Jeudy G, Skowron F, Aubin F, Visseaux L, Mansard S, Dereure O, Lesage FX, Guillot B. French Group of Onco-Dermatology. J Immunother. 2019;42(5):175–9.
Govindaraj C, Madondo M, Kong YY, Tan P, Wei A, Plebanski M. Lenalidomide-based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am J Hematol. 2014;89(8):795–802. https://doi.org/10.1002/ajh.23746.
van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, Nowak AK, Lake RA. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58(8):1219–28. https://doi.org/10.1007/s00262-008-0628-9.
Giannopoulos K, Dmoszynska A, Kowal M, Wasik-Szczepanek E, Bojarska-Junak A, Rolinski J, Döhner H, Stilgenbauer S, Bullinger L. Thalidomide exerts distinct molecular antileukemic effects and combined thalidomide/fludarabine therapy is clinically effective in high-risk chronic lymphocytic leukemia. Leukemia. 2009;23(10):1771–8. https://doi.org/10.1038/leu.2009.98.
Wei X, Gong J, Zhu J, Wang P, Li N, Zhu W, Li J. The suppressive effect of triptolide on chronic colitis and TNF-alpha/TNFR2 signal pathway in interleukin-10 deficient mice. Clinical Immunol. 2008;129(2):211–8. https://doi.org/10.1016/j.clim.2008.07.018.
Liu B, Zhang H, Li J, Lu C, Chen G, Zhang G, Lu A, He X. Triptolide downregulates TREG cells and the level of IL-10, TGF-β, and VEGF in melanoma-bearing mice. Planta Med. 2013;79(15):1401–7. https://doi.org/10.1055/s-0033-1350708.
Grell M, Becke FM, Wajant H, Männel DN, Scheurich P. Tumor necrosis factor (TNF) receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28(1):257–63. https://doi.org/10.1002/(SICI)1521-4141(199801)28:01%3c257::AID-IMMU257%3e3.0.CO;2-G.
Lamontain V, Schmid T, Weber-Steffens D, Zeller D, Jenei-Lanzl Z, Wajant H, Straub RH, Männel DN. Stimulation of TNF receptor type 2 expands regulatory T cells and ameliorates established collagen-induced arthritis in mice. Cell Mol Immunol. 2019;16(1):65–74. https://doi.org/10.1038/cmi.2017.138.
Acknowledgements
N/A
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author declares no conflict of interest or competing interest.
Ethical approval
Not Applicable
Informed consent
N/A
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quazi, S. TNFR2 antagonist and agonist: a potential therapeutics in cancer immunotherapy. Med Oncol 39, 215 (2022). https://doi.org/10.1007/s12032-022-01772-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01772-2