Abstract
Introduction
This research aims to optimize and predict the effectiveness of imatinib mesylate (imatinib) in tumors expressing platelet-derived growth factors (PDGF-AA, BB), kit/stem cell factor (SCF) ligands and their respective receptors (PDGFR-α, PDGFR-β, and c-kit).
Material and Methods
Samples of normal primary human T cells were incubated with graded concentrations of 1–5 μM imatinib. The energy yield by imatinib doses in those samples was identified in H-thymidine proliferation assay as described before in earlier studies. Tumor models of human pancreatic adenocarcinoma L3.6pl (PDGFAA/PDGFR-α-positive and KIT-negative), human male gonad Leydig tumor cells MA10 (PDGF-AA/PDGFR-α- positive and KIT-positive), human small-cell lung cancer [H209 (KIT-positive), NCI-H526 (PDGFR β-positive and KIT-positive), and NCI-H82 (PDGFR β-positive and KIT-negative)], and human neuroblastoma SMS-KCNR (PDGF-BB/PDGFR-β-positive and KIT-positive) in athymic nude mice were used. The antitumor activity of different doses of imatinib in different regimens in those xenografts was predicted as described before in earlier studies.
Results
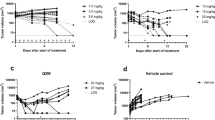
The energy yield by drug doses was perfectly logarithmic correlated (r = 1) with the drug dose. An efficient dose-energy model with perfect fit (R = 1) estimating the energy yield by imatinib doses has been established to administer the personalized dose. Predictions for the antitumor activity of imatinib in those xenografts using the dose-energy model and the histologic grade of the control animals were 100 % identical to those actually induced.
Conclusion
The effect of imatinib is transient and reversible, reduces tyrosine phosphorylation of tumor-derived PDGFR-α, PDGFR-β, and c-kit without affecting their levels of expression. A resumption of tumor growth nearly identical to the growth prior to therapy should be expected whenever the treatment is stopped. Tumors of PDGF-AA/PDGFR-α exhibit significant resistance to imatinib which requires administering imatinib three times a day, whereas resistance of tumors of PDGF-BB/PDGFR-β or KIT-positive is relatively lower which requires administering imatinib two times a day only to produce an actual inhibition 100 % identical to that predicted for tumor growth.

Similar content being viewed by others
References
Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74(10):589–607.
Crespo O, Kang SC, Daneman R, Lindstrom TM, Ho PP, Sobel RA, et al. Tyrosine kinase inhibitors ameliorate autoimmune encephalomyelitis in a mouse model of multiple sclerosis. J Clin Immunol. 2011. doi:10.1007/s10875-011-9579-6.
Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–42.
Moawad EY. Induction of Multiple Sclerosis and Response to Tyrosine Kinase Inhibitors. Ind J Clin Biochem. 2014;29(4):491–5.
Emad Y. Moawad (2013). Induction of rheumatoid arthritis and response to tyrosine kinase inhibitors. Universal Journal of Medical Science, 1 , 50–55. doi: 10.13189/ujmsj.2013.010205. http://www.hrpub.org/journals/article_info.php?aid=416
Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–45.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–32.
Buchdunger E, Zimmermann J, Mett H, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–4.
Okuda K, Weisberg E, Gilliland DG, Griffin JD. ARG tyrosine kinase activity is inhibited by STI571. Blood. 2001;97:2440–8.
Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20:1692–703.
Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52.
O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6.
DeMatteo RP. The GIST of targeted cancer therapy: a tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571). Ann Surg Oncol. 2002;9:831–9.
Cortes J, O’Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104(7):2204–5.
Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502.
Beppu K, Jaboine J, Merchant MS, Mackall CL, Thiele CJ. Effect of imatinib mesylate on neuroblastoma tumorigenesis and vascular endothelial growth factor expression. J Natl Cancer Inst. 2004;96:46–55.
Merchant MS, Woo CW, Mackall CL, Thiele CJ. Potential use of imatinib in Ewing’s Sarcoma: evidence for in vitro and in vivo activity. J Natl Cancer Inst. 2002;94:1673–9.
Krystal GW, Honsawek S, Litz J, Buchdunger E. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res. 2000;6:3319–26.
Moawad E. Isolated system towards a successful radiotherapy treatment. Nucl Med Mol Imaging. 2010;44:123–36. Free PMC Article.
Moawad EY. Radiotherapy and risks of tumor regrowth or inducing second cancer. Cancer Nanotechnol. 2011;2:81–93.
Emad Y. Moawad (2013). Safe doses and cancer treatment evaluation. Cancer and Oncology Research, 1 , 6–11. doi: 10.13189/cor.2013.010102. http://www.hrpub.org/journals/article_info.php?aid=48
Emad Y. Moawad (2013). Pathologic cancer staging by measuring cell growth energy. Cancer and Oncology Research, 1 , 69–74. doi: 10.13189/cor.2013.010301. http://www.hrpub.org/journals/article_info.php?aid=660
Emad Y. Moawad (2013). The mechanism by which chronic myeloid leukemia responds to interferon-α treatment. Advances in Pharmacology and Pharmacy, 1 , 88–94. doi: 10.13189/app.2013.010207. http://www.hrpub.org/journals/article_info.php?aid=451
Moawad EY. Clinical and pathological staging of the cancer at the nanoscale. Cancer Nano. 2012;3:37–46.
Moawad EY. Reconciliation between the clinical and pathological staging of cancer. Comp Clin Pathol. 2014;23:255–62.
Moawad EY. Administering the optimum dose of L-arginine in regional tumor therapy. Ind J Clin Biochem. 2014;29(4):442–51.
Moawad EY. Identifying the optimal dose of ritonavir in the treatment of malignancies. Metab Brain Dis. 2014;29:533–40.
Moawad EY. Optimal standard regimen and predicting response to docetaxel therapy. Mutat Res-Fundam Mol Mech Mutagen. 2014;770:120–7.
Emad Y. Moawad (2013). Safe cancer screening for patients after lumpectomy, survivors, and healthy subjects. Cancer and Oncology Research, 1 , 15–23. doi: 10.13189/cor.2013.010201. http://www.hrpub.org/journals/article_info.php?aid=281
Emad Y. Optimizing bioethanol production through regulating yeast growth energy. Syst Synth Biol. 2012;6:61–8. Free PMC Article.
Emad Y. Moawad (2013). Growth energy of bacteria and the associated electricity generation in fuel cells. Bioengineering and Bioscience, 1 , 5–10. doi: 10.13189/bb.2013.01010 http://www.hrpub.org/journals/article_info.php?aid=129
Emad Y. Moawad (2013). Nuclear transmutation and cancer in the biological cell. International Journal of Biochemistry and Biophysics, 1 , 1–8. doi: 10.13189/ijbb.2013.010101. http://www.hrpub.org/journals/article_info.php?aid=139
Emad Y. Cell growth energy represents a measure for man health; regulates nuclear transmutations and aberrant activation in human cell. Univ J Med Sci. 2013;1:27–35. doi:10.13189/ujmsj.2013.010203. http://www.hrpub.org/journals/article_info.php?aid=414.
Emad Y. Moawad (2013). Purification of sewage water through the protection of the environment from radioactive contamination. Energy and Environmental Engineering, 1 , 55–61. doi: 10.13189/eee.2013.010203. http://www.hrpub.org/journals/article_info.php?aid=304
Moawad, E. Y. (2015). Mass-energy conversion in the decaying system and doubling time-energy conversion in the biological system. Journal of Physics Research and Reviews, 1(1), 1–13. http://chromejournals.com/sci/index.php/JPRR/article/view/5
Kyprianou N, English HF, Davidson NE, Isaacs JT. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991;51(1):162–6.
Steel GG. Growth kinetics of tumours. Cell population kinetics in relation to the growth and treatment of cancer. Oxford: Clarendon Press; 1977.
Dietz AB et al. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104:1094–9.
Hwang RF, Yokoi K, Bucana CD, Tsan R, Killion JJ, Evans DB, et al. Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res. 2003;9(17):6534–44.
Basciani S, Brama M, Mariani S, De Luca G, Arizzi M, Vesci L, et al. Imatinib mesylate inhibits Leydig cell tumor growth: evidence for in vitro and in vivo activity. Cancer Res. 2005;65(5):1897–903.
Wolff NC, Randle DE, Egorin MJ, Minna JD, Ilaria RL. Imatinib mesylate efficiently achieves therapeutic intratumor concentrations in vivo but has limited activity in a xenograft model of small cell lung cancer. Clin Cancer Res. 2004;10(10):3528–34.
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor β in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2(5):471–8.
Kenneth Barbalace. Periodic Table of Elements - H - Hydrogen. EnvironmentalChemistry.com. 1995–2011. Accessed on-line: 12/21/2011http://EnvironmentalChemistry.com/yogi/periodic/H-pg2.html
Zhang L, Yang N, Katsaros D, et al. The oncogene phosphatidylinositol 3 V-kinase catalytic subunit a promotes angiogenesis via vascular endothelial growth factor in ovarian carcinoma. Cancer Res. 2003;63:4225–31.
Emad Y. Moawad, Identifying and predicting the effectiveness of carboplatin in vivo and in vitro and evaluating its combination with paclitaxel. Indian J Gynecol Oncolog. 2015;13:1-9.
Gambacorti-Passerini C, Barni R, le Coutre P, et al. Role of a1 acid glycoprotein in the in vivo resistance of human BCR-ABL(+) leukemic cells to the abl inhibitor STI571. J Natl Cancer Inst. 2000;92:1641–50.
Cohen PS, Chan JP, Lipkunskaya M, Biedler JL, Seeger RC. Expression of stem cell factor and c-kit in human neuroblastoma. Blood. 1994;84:3465–72.
Timeus F, Crescenzio N, Valle P, Pistamiglio P, Piglione M, Garelli E, et al. Stem cell factor suppresses apoptosis in neuroblastoma cell lines. Exp Hematol. 1997;25:1253–60.
Gschwind, Hans-Peter, Pfaar U, Waldmeier F, Zollinger M, Sayer C, et al. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab Dispos. 2005;33(10):1503–12.
Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–62.
Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. Free Full Text | Web of Science |.
Berman, Ellin, Nicolaides M, Maki RG, Fleisher M, Chanel S, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354(19):2006–13.
Conflict of Interest
The author declares that there is no conflict of interest concerning this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Emad Y. Moawad is a member of the Korean Society of Nuclear Medicine
Rights and permissions
About this article
Cite this article
Moawad, E.Y. Predicting Effectiveness of Imatinib Mesylate in Tumors Expressing Platelet-Derived Growth Factors (PDGF-AA, PDGF-BB), Stem Cell Factor Ligands and Their Respective Receptors (PDGFR-α, PDGFR-β, and c-kit). J Gastrointest Canc 46, 272–283 (2015). https://doi.org/10.1007/s12029-015-9721-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-015-9721-4