Abstract
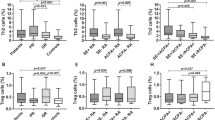
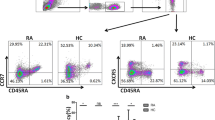
In rheumatoid arthritis (RA), immune homeostasis is maintained by T regulatory cells (Tregs) that in an inflammatory milieu can change towards T-helper-like phenotypes (Th-like Tregs). Our aim was to examine the phenotypic and functional characteristics of CD4+CD25+CD127lo/− Tregs, Th-like Tregs and T effector (Teff) cells in the peripheral blood (PB) and synovial fluid (SF) of treatment-naïve early RA, as compared to osteoarthritis (OA) and healthy control (HC) peripheral blood. Frequencies of Tregs, CXCR3, CCR6 expressing Tregs (Th-like Tregs), and Teff cells were analyzed using flow cytometry in RA (n = 80), OA (n = 20), and HC (n = 40). Cytokine concentrations of the respective T cell subsets in plasma and SF were measured using flow cytometric bead array. Tregs sorted from RA and HC PB using magnetic beads were analyzed for functional capacities by CFSE proliferation assay and FOXP3 gene expression using real-time PCR. We observed that the frequencies of Th17 cells in PB and SF were significantly higher in RA when compared to HC, whereas Tregs were lower in PB and high in SF compared to HC and OA respectively. Th1- and Th17-related pro-inflammatory cytokines IL12p70, INF-γ, TNF-α, and IL-6, and IL-17A were significantly higher in the plasma and SF of RA. Tregs expressing CXCR3 (Th1-like Tregs) and CCR6 (Th17-like Treg) were significantly higher in PB and SF of RA compared to controls and was positively associated with seropositivity and disease activity. Treg cells isolated from peripheral blood of RA showed decreased function and reduced FOXP3 gene expression compared to HC. In our study, we have demonstrated higher frequencies of Th1 and Th17 cells and increased circulatory and SF pro-inflammatory cytokines (IL12P70, INF-γ, IL-6, IL-17A, and TNF-α) in RA. This inflammatory milieu might alter total Tregs frequencies and influence conversion of Tregs into Th-like Tregs.





Similar content being viewed by others
Data Availability
Data and materials are available upon request.
References
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. https://doi.org/10.1038/nature01661.
Morita Y, Yamamura M, Kawashima M, et al. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669–76. https://doi.org/10.1002/1529-0131(199809)41:9%3c1669:AID-ART19%3e3.0.CO;2-G.
Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466–71.
James EA, Rieck M, Pieper J, et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014;66:1712–22. https://doi.org/10.1002/art.38637.
Aldridge J, Ekwall A-KH, Mark L, et al. T helper cells in synovial fluid of patients with rheumatoid arthritis primarily have a Th1 and a CXCR3+Th2 phenotype. Arthritis Res Ther. 2020;22:245. https://doi.org/10.1186/s13075-020-02349-y.
Henriques A, Gomes V, Duarte C, et al. Distribution and functional plasticity of peripheral blood Th(c)17 and Th(c)1 in rheumatoid arthritis. Rheumatol Int. 2013;33:2093–9. https://doi.org/10.1007/s00296-013-2703-6.
van Amelsfort JMR, Jacobs KMG, Bijlsma JWJ, et al. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. https://doi.org/10.1002/art.20499.
Cao D, van Vollenhoven R, Klareskog L, et al. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335-346. https://doi.org/10.1186/ar1192.
Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. https://doi.org/10.1093/intimm/dxm014.
Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. https://doi.org/10.1084/jem.20060772.
Koenen HJPM, Smeets RL, Vink PM, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. https://doi.org/10.1182/blood-2008-01-133967.
Voo KS, Wang Y-H, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–8. https://doi.org/10.1073/pnas.0900408106.
Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–65. https://doi.org/10.1053/j.gastro.2010.12.002.
Duhen T, Duhen R, Lanzavecchia A, et al. Functionally distinct subsets of human FOXP3+ treg cells that phenotypically mirror effector Th cells (Blood (2012) 119, 19 (4430–4440)). Blood. 2012;119:4430–40. https://doi.org/10.1182/blood-2011-11-392324.
Beriou G, Costantino CM, Ashley CW, et al. IL-17–producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. https://doi.org/10.1182/blood-2008-10-183251.
Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORγt+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–7. https://doi.org/10.1073/pnas.1008247107.
Zhang R, Miao J, Zhang K, et al. Th1-like Treg cells are increased but deficient in function in rheumatoid arthritis. Front Immunol. 2022;13:863753. https://doi.org/10.3389/fimmu.2022.863753.
Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–5. https://doi.org/10.1038/nm.2389.
McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–26. https://doi.org/10.4049/jimmunol.1003099.
Lawson CA, Brown AK, Bejarano V, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford). 2006;45:1210–7. https://doi.org/10.1093/rheumatology/kel089.
Lina C, Conghua W, Nan L, Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:596–605. https://doi.org/10.1007/s10875-011-9542-6.
Aldridge J, Pandya JM, Meurs L, et al. Sex-based differences in association between circulating T cell subsets and disease activity in untreated early rheumatoid arthritis patients. Arthritis Res Ther. 2018;20:150. https://doi.org/10.1186/s13075-018-1648-2.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. https://doi.org/10.1002/art.27584.
Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–9. https://doi.org/10.1136/ard.2009.113100.
Penatti A, Facciotti F, De Matteis R, et al. Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2017;19:103. https://doi.org/10.1186/s13075-017-1305-1.
Yu N, Li X, Song W, et al. CD4(+)CD25 (+)CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35:1773–80. https://doi.org/10.1007/s10753-012-9496-8.
Tu J, Huang W, Zhang W, et al. Two main cellular components in rheumatoid arthritis: communication between T cells and fibroblast-like synoviocytes in the Joint Synovium. Front Immunol. 2022;13:922111. https://doi.org/10.3389/fimmu.2022.922111.
Furuzawa-Carballeda J, Lima G, Jakez-Ocampo J, Llorente L. Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: a cross-sectional study. Eur J Clin Invest. 2011;41:1037–46. https://doi.org/10.1111/j.1365-2362.2011.02491.x.
Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–8. https://doi.org/10.1038/nm.3085.
Qiu R, Zhou L, Ma Y, et al. Regulatory T cell plasticity and stability and autoimmune diseases. Clin Rev Allergy Immunol. 2020;1:52–70.
van Hamburg JP, Asmawidjaja PS, Davelaar N, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. https://doi.org/10.1002/art.30093.
Wang T, Sun X, Zhao J, et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74:1293–301. https://doi.org/10.1136/annrheumdis-2013-204228.
Paradowska-Gorycka A, Wajda A, Romanowska-Próchnicka K, et al. Th17/Treg-related transcriptional factor expression and cytokine profile in patients with rheumatoid arthritis. Front Immunol. 2020;11:572858. https://doi.org/10.3389/fimmu.2020.572858.
Moradi B, Schnatzer P, Hagmann S, et al. CD4+CD25+/highCD127low/- regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints—analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther. 2014;16:R97. https://doi.org/10.1186/ar4545.
Kawashiri S-Y, Kawakami A, Okada A, et al. CD4+CD25(high)CD127(low/-) Treg cell frequency from peripheral blood correlates with disease activity in patients with rheumatoid arthritis. J Rheumatol. 2011;38:2517–21. https://doi.org/10.3899/jrheum.110283.
Wang W, Shao S, Jiao Z, et al. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int. 2012;32:887–93. https://doi.org/10.1007/s00296-010-1710-0.
Gao N, Cui W, Zhao LM, et al. Contribution of Th2-like Treg cells to the pathogenesis of Takayasu’s arteritis. Clin Exp Rheumatol. 2020;38(Suppl 124):48–54.
Li N, Wei W, Yin F, et al. The abnormal expression of CCR4 and CCR6 on Tregs in rheumatoid arthritis. Int J Clin Exp Med. 2015;8:15043–53.
Kim CH. Migration and function of Th17 cells. Inflamm Allergy Drug Targets. 2009;3:221–8.
Nevius E, Gomes AC, Pereira JP. A comprehensive review of inflammatory cell migration in rheumatoid arthritis. Clin Rev Allergy Immunol. 2016;51:59–78. https://doi.org/10.1007/s12016-015-8520-9.
Mellado M, Martínez-Muñoz L, Cascio G, et al. T cell migration in rheumatoid arthritis. Front Immunol. 2015;6:384. https://doi.org/10.3389/fimmu.2015.00384.
Paulissen SMJ, van Hamburg JP, Dankers W, Lubberts E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine. 2015;74:43–53. https://doi.org/10.1016/j.cyto.2015.02.002.
Jiang Q, Yang G, Liu Q, et al. Function and role of regulatory T cells in rheumatoid arthritis. Front Immunol. 2021;12:1021. https://doi.org/10.3389/fimmu.2021.626193.
Alvandpur N, Tabatabaei R, Tahamoli-Roudsari A, et al. Circulating IFN-γ producing CD4+ T cells and IL-17A producing CD4+ T cells, HLA-shared epitope and ACPA may characterize the clinical response to therapy in rheumatoid arthritis patients. Hum Immunol. 2020;81:228–36. https://doi.org/10.1016/j.humimm.2020.02.008.
Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. https://doi.org/10.1186/ar1733.
Lindqvist E, Eberhardt K, Bendtzen K, et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;64:196–201. https://doi.org/10.1136/ard.2003.019992.
Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Investig. 2006;116:961–73. https://doi.org/10.1172/JCI25422
Liu Z, Gu J, Qin Z, et al. Decreased Foxp3 and function of Tregs caused immune imbalance and liver injury in patients with autoimmune liver diseases post-liver transplantation. Ann Transl Med. 2020;8:534–534. https://doi.org/10.21037/atm.2020.03.203.
Jj G, F F, Mm C, et al (2015) Naive T cell maintenance and function in human aging. Journal of immunology (Baltimore, Md : 1950) 194 https://doi.org/10.4049/jimmunol.1500046
Funding
The work was supported by Department of Science and Technology-Science and Engineering Research Board (DST-SERB), India (SERB/F/0253/2016–17), and JIPMER Intramural Research Fund (JIP/Res/Intra-PhD/01/2014–15 and JIP/Res/Intra-PhD/O2/2015–16).
Author information
Authors and Affiliations
Contributions
VSN, KV, and CMM contributed to the conception and the design of the study. VSN and CKG recruited patients and collected clinical data. KV performed experiments, analyzed the data, and drafted the manuscript. SNB was involved in experiments and statistical analyses. MTT, CMM, CKG, and PBN contributed to interpretation of the data, carefully read the article, and suggested edits. VSN, CMM, and MTT critically revised the article for important intellectual content. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the JIPMER institute ethics committee and conducted the following Principles of the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Protocol No. JIP/IEC/2014/10/483 dated 30.01.2015.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kommoju, V., Mariaselvam, C.M., Bulusu, S.N. et al. Conventional Tregs in treatment-naïve rheumatoid arthritis are deficient in suppressive function with an increase in percentage of CXCR3 and CCR6 expressing Tregs. Immunol Res (2023). https://doi.org/10.1007/s12026-023-09444-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-023-09444-7