Abstract
Purpose
Angiogenic markers in neuroendocrine neoplasms (NENs) have recently received increasing attention, but their clinical role remains unclear. The aim of this study was to evaluate the role of angiogenic markers in NEN aggressiveness and prognosis.
Methods
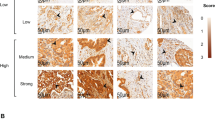
We performed a prospective observational study including 46 consecutive patients with proven NENs of pulmonary (45.65%) and gastro-entero-pancreatic (GEP) (54.35%) origin and 29 healthy controls. Circulating pro-angiogenic factors were measured by ELISA assay. ANG2 tissue expression was evaluated in a subgroup of ten patients by immunohistochemistry.
Results
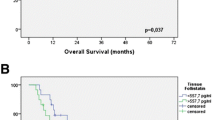
The study demonstrated a significantly higher level of ANG2, ANG1, sTIE2, and PROK2 in patients affected by NENs compared to controls. In the NENs’ group we measured that: (i) ANG2 levels were higher in poorly vs well-differentiated NENs: 4.85 (2.75–7.42) vs 3.16 (1.66–6.36) ng/ml, p = 0.046 and in tumor stage 3–4 compared to stage 1–2: 4.24 (2.66–8.72) vs 2.73 (1.53–5.70), p = 0.044; (ii) ANG2 and PROK2 were significantly higher in patents with progressive disease compared to stable disease: ANG2 = 6.26 (3.98–10.99) vs 2.73 (1.65–4.36) pg/ml, p = 0.001; PROK2 = 29.19 (28.42–32.25) vs 28.37 (28.14–28.91) pg/ml, p = 0.035. Immunohistochemistry confirmed ANG2 expression in tumor specimens.
Conclusions
We documented higher levels of angiogenic markers in NENs, with an association between ANG2 serum levels and NENs morphology and staging. In both GEP and lung NENs, ANG2 and PROK2 are higher in case of tumor progression, suggesting a potential role as prognostic markers in NENs patients.




Similar content being viewed by others
Data availability
Data are available from the authors.
Abbreviations
- NEN:
-
neuroendocrine neoplasm
- NET:
-
neuroendocrine tumor
- NEC:
-
neuroendocrine carcinoma
- GEP:
-
gastro-entero-pancreatic
- ANG:
-
angiopoietin
- SSA:
-
somatostatin analogs
- VEGF:
-
vascular endothelial growth factor
- PROK:
-
prokineticins
- PROKR:
-
prokineticins receptor
- sTIE2:
-
soluble TIE2
- SD:
-
standard deviation
References
J. Hallet, C.H. Law, M. Cukier, R. Saskin, N. Liu, S. Singh, Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121(4), 589–597 (2015). https://doi.org/10.1002/cncr.29099
A. Dasari, C. Shen, D. Halperin, B. Zhao, S. Zhou, Y. Xu et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3(10), 1335–1342 (2017). https://doi.org/10.1001/jamaoncol.2017.0589
K.I. Alexandraki, M. Tsoli, G. Kyriakopoulos, A. Angelousi, G. Nikolopoulos, D. Kolomodi et al. Current concepts in the diagnosis and management of neuroendocrine neoplasms of unknown primary origin. Minerva Endocrinol. 44(4), 378–386 (2019). https://doi.org/10.23736/S0391-1977.19.03012-8
G. Gaudenzi, A. Dicitore, S. Carra, D. Saronni, C. Pozza, E. Giannetta et al. MANAGEMENT OF ENDOCRINE DISEASE: precision medicine in neuroendocrine neoplasms: an update on current management and future perspectives. Eur. J. Endocrinol. 181(1), R1–R10 (2019). https://doi.org/10.1530/EJE-19-0021
A. Couvelard, D. O’Toole, H. Turley, R. Leek, A. Sauvanet, C. Degott et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br. J. Cancer 92(1), 94–101 (2005). https://doi.org/10.1038/sj.bjc.6602245
M. Theodoropoulou, G.K. Stalla, Somatostatin receptors: from signaling to clinical practice. Front. Neuroendocrinol. 34(3), 228–252 (2013). https://doi.org/10.1016/j.yfrne.2013.07.005
C. Viallard, B. Larrivee, Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20(4), 409–426 (2017). https://doi.org/10.1007/s10456-017-9562-9
M. Thomas, H.G. Augustin, The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 12(2), 125–137 (2009). https://doi.org/10.1007/s10456-009-9147-3
D. Chakroborty, C. Sarkar, H. Yu, J. Wang, Z. Liu, P.S. Dasgupta et al. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc. Natl Acad. Sci. USA. 108(51), 20730–20735 (2011). https://doi.org/10.1073/pnas.1108696108
J. Holash, S.J. Wiegand, G.D. Yancopoulos, New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18(38), 5356–5362 (1999). https://doi.org/10.1038/sj.onc.1203035
H.G. Augustin, G.Y. Koh, G. Thurston, K. Alitalo, Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell. Biol. 10(3), 165–177 (2009). https://doi.org/10.1038/nrm2639
L. Negri, R. Lattanzi, E. Giannini, P. Melchiorri, Bv8/Prokineticin proteins and their receptors. Life Sci. 81(14), 1103–1116 (2007). https://doi.org/10.1016/j.lfs.2007.08.011
E.S. Ngan, P.K. Tam, Prokineticin-signaling pathway. Int. J. Biochem. Cell. Biol. 40(9), 1679–1684 (2008). https://doi.org/10.1016/j.biocel.2008.03.010
Y. Zhao, J. Wu, X. Wang, H. Jia, D.N. Chen, J.D. Li, Prokineticins and their G protein-coupled receptors in health and disease. Prog. Mol. Biol. Transl. Sci. 161, 149–179 (2019). https://doi.org/10.1016/bs.pmbts.2018.09.006
N. Figueroa-Vega, A. Diaz, M. Adrados, C. Alvarez-Escola, A. Paniagua, J. Aragones et al. The association of the angiopoietin/Tie-2 system with the development of metastasis and leukocyte migration in neuroendocrine tumors. Endocr. Relat. Cancer 17(4), 897–908 (2010). https://doi.org/10.1677/ERC-10-0020
A.S. Corlan, A.M. Cimpean, A.A. Jitariu, E. Melnic, M. Raica, Endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in cancer development and tumor angiogenesis. Int. J. Endocrinol. 2017, 3232905 (2017). https://doi.org/10.1155/2017/3232905
R. Rust, C. Gantner, M.E. Schwab, Pro- and antiangiogenic therapies: current status and clinical implications. FASEB J. 33(1), 34–48 (2019). https://doi.org/10.1096/fj.201800640RR
H. Choi, A. Moon, Crosstalk between cancer cells and endothelial cells: implications for tumor progression and intervention. Arch. Pharm. Res. 41(7), 711–724 (2018). https://doi.org/10.1007/s12272-018-1051-1
J. Folkman, Tumor angiogenesis. Adv. Cancer Res. 19, 331–358 (1974). https://doi.org/10.1016/s0065-230x(08)60058-5
R.I. Teleanu, C. Chircov, A.M. Grumezescu, D.M. Teleanu. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 9(1), 84 (2020). https://doi.org/10.3390/jcm9010084.
A.M. Isidori, M.A. Venneri, D. Fiore, Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J. Endocrinol. Invest. 39(11), 1235–1246 (2016). https://doi.org/10.1007/s40618-016-0502-0
M.A. Venneri, F. Barbagallo, D. Fiore, R. De Gaetano, E. Giannetta, E. Sbardella et al. PDE5 inhibition stimulates Tie2-expressing monocytes and Angiopoietin-1 restoring angiogenic homeostasis in diabetes. J. Clin. Endocrinol. Metab. 104(7), 2623–2636 (2019). https://doi.org/10.1210/jc.2018-02525
R. Lorbeer, S.E. Baumeister, M. Dorr, S.B. Felix, M. Nauck, A. Grotevendt et al. Angiopoietin-2, its soluble receptor Tie-2 and subclinical cardiovascular disease in a population-based sample. Heart 101(3), 178–184 (2015). https://doi.org/10.1136/heartjnl-2014-306056
M. Whitehead, A. Osborne, P.S. Widdowson, P. Yu-Wai-Man, K.R. Martin, Angiopoietins in diabetic retinopathy: current understanding and therapeutic potential. J. Diabetes Res. 2019, 5140521 (2019). https://doi.org/10.1155/2019/5140521
J. Sahni, S.S. Patel, P.U. Dugel, A.M. Khanani, C.D. Jhaveri, C.C. Wykoff et al. Simultaneous inhibition of Angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology 126(8), 1155–1170 (2019). https://doi.org/10.1016/j.ophtha.2019.03.023
H. Huang, A. Bhat, G. Woodnutt, R. Lappe, Targeting the ANGPT-TIE2 pathway in malignancy. Nat. Rev. Cancer 10(8), 575–585 (2010). https://doi.org/10.1038/nrc2894
L. Hakanpaa, T. Sipila, V.M. Leppanen, P. Gautam, H. Nurmi, G. Jacquemet et al. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat. Commun. 6, 5962 (2015). https://doi.org/10.1038/ncomms6962
K.M. Detjen, S. Rieke, A. Deters, P. Schulz, A. Rexin, S. Vollmer et al. Angiopoietin-2 promotes disease progression of neuroendocrine tumors. Clin. Cancer Res. 16(2), 420–429 (2010). https://doi.org/10.1158/1078-0432.CCR-09-1924
R. Srirajaskanthan, G. Dancey, A. Hackshaw, T. Luong, M.E. Caplin, T. Meyer, Circulating angiopoietin-2 is elevated in patients with neuroendocrine tumours and correlates with disease burden and prognosis. Endocr. Relat. Cancer 16(3), 967–976 (2009). https://doi.org/10.1677/ERC-09-0089
G. Melen-Mucha, A. Niedziela, S. Mucha, E. Motylewska, H. Lawnicka, J. Komorowski et al. Elevated peripheral blood plasma concentrations of tie-2 and angiopoietin 2 in patients with neuroendocrine tumors. Int. J. Mol. Sci. 13(2), 1444–1460 (2012). https://doi.org/10.3390/ijms13021444
J. LeCouter, J. Kowalski, J. Foster, P. Hass, Z. Zhang, L. Dillard-Telm et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412(6850), 877–884 (2001). https://doi.org/10.1038/35091000
M. Li, C.M. Bullock, D.J. Knauer, F.J. Ehlert, Q.Y. Zhou, Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol. 59(4), 692–698 (2001). https://doi.org/10.1124/mol.59.4.692
F.A. Ferrer, L.J. Miller, R.I. Andrawis, S.H. Kurtzman, P.C. Albertsen, V.P. Laudone et al. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology 51(1), 161–167 (1998). https://doi.org/10.1016/s0090-4295(97)00491-3
T. Goi, M. Fujioka, Y. Satoh, S. Tabata, K. Koneri, H. Nagano et al. Angiogenesis and tumor proliferation/metastasis of human colorectal cancer cell line SW620 transfected with endocrine glands-derived-vascular endothelial growth factor, as a new angiogenic factor. Cancer Res. 64(6), 1906–1910 (2004). https://doi.org/10.1158/0008-5472.can-3696-2
D. Pasquali, V. Rossi, S. Staibano, G. De Rosa, P. Chieffi, D. Prezioso et al. The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinology 147(9), 4245–4251 (2006). https://doi.org/10.1210/en.2006-0614
J. Monnier, M. Samson, Prokineticins in angiogenesis and cancer. Cancer Lett. 296(2), 144–149 (2010). https://doi.org/10.1016/j.canlet.2010.06.011
N. Ferrara, Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr. Opin. Hematol. 17(3), 219–224 (2010). https://doi.org/10.1097/MOH.0b013e3283386660
Y. Wang, X. Guo, H. Ma, L. Lu, R. Zhang, Prokineticin-2 is associated with metabolic syndrome in a middle-aged and elderly Chinese population. Lipids Health Dis. 15, 1 (2016). https://doi.org/10.1186/s12944-015-0172-5
T. Schirinzi, D. Maftei, M. Pieri, S. Bernardini, N.B. Mercuri, R. Lattanzi et al. Increase of Prokineticin-2 in serum of patients with Parkinson’s disease. Mov. Disord. 36(4), 1031–1033 (2021). https://doi.org/10.1002/mds.28458
T. Collot, J.D. Fumet, Q. Klopfenstein, J. Vincent, L. Bengrine, F. Ghiringhelli, Bevacizumab-based chemotherapy for poorly-differentiated neuroendocrine tumors. Anticancer Res. 38(10), 5963–5968 (2018). https://doi.org/10.21873/anticanres.12943
J.C. Yao, A. Phan, P.M. Hoff, H.X. Chen, C. Charnsangavej, S.C. Yeung et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J. Clin. Oncol. 26(8), 1316–1323 (2008). https://doi.org/10.1200/JCO.2007.13.6374
J.C. Yao, K.A. Guthrie, C. Moran, J.R. Strosberg, M.H. Kulke, J.A. Chan et al. Phase III prospective randomized comparison trial of depot octreotide plus interferon Alfa-2b versus depot octreotide plus bevacizumab in patients with advanced carcinoid tumors: SWOG S0518. J. Clin. Oncol. 35(15), 1695–1703 (2017). https://doi.org/10.1200/JCO.2016.70.4072
M. Kulke, D. Niedzwiecki, N.R. Foster, B. Fruth, P. Kunz, H. Kennecke et al. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). J. Clin. Oncol. 33, 2015 (2015).
J. Gillen, D. Richardson, K. Moore, Angiopoietin-1 and Angiopoietin-2 inhibitors: clinical development. Curr. Oncol. Rep. 21(3), 22 (2019). https://doi.org/10.1007/s11912-019-0771-9
N. Rigamonti, E. Kadioglu, I. Keklikoglou, C. Wyser Rmili, C.C. Leow, M. De Palma, Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell. Rep. 8(3), 696–706 (2014). https://doi.org/10.1016/j.celrep.2014.06.059
V. Baeriswyl, G. Christofori, The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 19(5), 329–337 (2009). https://doi.org/10.1016/j.semcancer.2009.05.003
K. Oberg, E. Krenning, A. Sundin, L. Bodei, M. Kidd, M. Tesselaar et al. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr. Connect. 5(5), 174–187 (2016). https://doi.org/10.1530/EC-16-0043
Acknowledgements
This work has been supported by the NETTARE Unit of Sapienza University. We would like to acknowledge all the members: Domenico Alvaro, Emanuela Anastasi, Antonio Angeloni, Oreste Bagni, Caterina Bangrazi, Massimiliano Bassi, Mario Bezzi, Nadia Bulzonetti, Vito Cantisani, Roberto Caronna, Giovanni Casella, Carlo Catalano, Roberta Centello, Enrico Cortesi, Ferdinando D’Ambrosio, Carlo Della Rocca, Adriano De Santis, Cira Di Gioia, Valentina Di Vito, Antongiulio Faggiano, Tiziana Feola, Daniele Gianfrilli, Alfredo Genco, Elisa Giannetta, Franco Iafrate, Andrea M. Isidori, Andrea Lenzi, Paolo Marchetti, Francesca Maccioni, Giulia Puliani, Carla Pandozzi, Franz Sesti, Carola Severi, Silverio Tomao, Vincenzo Tombolini, Federico Venuta, Monica Verrico.
Nettare Unit
Domenico Alvaro7, Emanuela Anastasi1, Antonio Angeloni1, Oreste Bagni8, Caterina Bangrazi9, Massimiliano Bassi10, Mario Bezzi11, Nadia Bulzonetti9, Vito Cantisani5, Roberto Caronna12, Giovanni Casella12, Carlo Catalano5, Roberta Centello1, Enrico Cortesi13, Ferdinando D’Ambrosio5, Carlo Della Rocca5, Adriano De Santis7, Cira Rosaria Tiziana Di Gioia5, Valentina Di Vito1, Antongiulio Faggiano6, Tiziana Feola1,4, Daniele Gianfrilli1, Alfredo Genco12, Elisa Giannetta1, Franco Iafrate5, Andrea M. Isidori1, Andrea Lenzi1, Paolo Marchetti13, Francesca Maccioni5, Alessio Molfino14, Maurizio Muscaritoli14, Carla Pandozzi1, Giulia Puliani1,2, Franz Sesti1, Carola Severi7, Silverio Tomao3, Vincenzo Tombolini9, Federico Venuta10, Monica Verrico3
Funding
This work was supported by the Ministerial research project PRIN2017Z3N3YC and Sapienza University Research Grant RM120172ADA2C4AF.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics approval
The study has been approved by the review board of Sapienza University of Rome (reference number 5917).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Puliani, G., Sesti, F., Anastasi, E. et al. Angiogenic factors as prognostic markers in neuroendocrine neoplasms. Endocrine 76, 208–217 (2022). https://doi.org/10.1007/s12020-021-02942-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02942-4