Abstract
Background
Despite multimodal therapy esophageal cancer often presents with poor prognosis. To improve outcome, tumor angiogenesis and anti-angiogenic therapeutic agents have recently gained importance. However, patient subgroups who benefit from anti-angiogenic therapy are not yet defined. In this retrospective exploratory study we investigated 9 angiogenic factors in patients’ serum and tissue samples with regard to their association with clinicopathological parameters, prognosis and response in patients with locally advanced preoperatively treated esophageal cancer.
Methods
From 2007 to 2012 preoperative serum and corresponding tumor tissue (n = 54), only serum (n = 20) or only tumor tissue (n = 4) were collected from esophageal squamous cell carcinoma (SCC) (n = 34) and adenocarcinoma of the esophagogastric junction (AEG) (n = 44) staged cT3/4NanyM0/x after preoperative chemo(radio)therapy. Angiogenic cytokine levels in both tissue and serum were measured by multiplex immunoassay.
Results
Median survival in all patients was 28.49 months. No significant difference was found in survival between SCC and AEG (p = 0.90). 26 patients were histopathological responders. Histopathological response was associated with prognosis (p = 0.05).
Angiogenic factors were associated with the following clinicopathological factors: tumor tissue expression of Angiopoietin-2 and Follistatin was higher in SCC compared to AEG (p = 0.022 and p = 0.001).
High HGF and Follistatin expression in the tumor tissue was associated with poor prognosis in all patients (p = 0.037 and p = 0.036). No association with prognosis was found in the patients’ serum. Neither patients’ serum nor tumor tissue showed an association between angiogenic factors and response to neoadjuvant therapy.
Conclusion
Two angiogenic factors (HGF and Follistatin) in posttherapeutic tumor tissue are associated with prognosis in esophageal cancer patients. Biological differences of AEG and SCC with respect to angiogenesis were evident by the different expression of 2 angiogenic factors. Results are promising and should be pursued prospectively, optimally sequentially pre- and posttherapeutically.
Similar content being viewed by others
Background
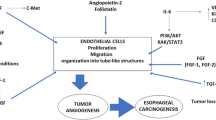
Esophageal cancer is known for its aggressive tumor growth and poor prognosis. 5-year overall survival rates vary between 15-25% [1]. This poor outcome is linked to the fact that the disease is often detected in an advanced state when dysphagia occurs, often making a cure by surgical resection difficult [2,3]. To improve this fatal situation, patients with locoregional disease receive neoadjuvant chemo- or chemoradiotherapy before undergoing surgery, which has been shown to provide a survival benefit [4-6]. Considering the fact that only patients who respond to this neoadjuvant therapy have a clear survival advantage, and that nonresponding patients do not, the prediction of response and prognosis is of highest interest [7-9]. Response rates differ depending on the chosen therapeutic regimen from 20–50% [7]. Even though different prediction algorithms exist, they are not yet used in clinical practice. Recent studies indicated that factors that shape the tumor microenvironment might influence patients’ response and outcome [10]. Tumor angiogenesis, the formation of new blood vessels within a tumor presents an important part of the tumor microenvironment. Beyond a certain size, tumors are not further supported by diffusion, but undergo an “angiogenic switch”, which supports further tumor growth and metastasis [11-13]. Angiogenesis within solid tumors is a complex process, involving many different factors that are active at different time points [14]. The significance of the resulting proangiogenic environment has led to the development of anti-angiogenic therapeutic agents against the main angiogenic factors. As first anti-angiogenic therapy an antibody, bevacizumab, was developed targeting against the prototypic angiogenic molecule vascular endothelial growth factor (VEGF-A). Addition of bevacizumab in metastatic colorectal cancers showed a survival benefit when added to Irinotecan and 5-Fluoruracile in a Phase III trial [15]. In Phase II studies the use of bevacizumab in patients with esophageal carcinoma in combination with Cisplatin and Capecitabine was associated with a higher response rate and longer progression-free survival. However, these trials failed to show a benefit of overall survival in patients with esophageal cancer [16]. In the REGARD trial it was recently shown that treatment with ramicirumab monotherapy, an antibody against VEGF receptor 2 (VEGFR-2), can prolong survival in patients with advanced gastric and esophageal cancer.
As a crucial element in esophageal cancer angiogenesis is linked to tumor growth and metastasis [17]. Angiogenic factors previously described in esophageal cancer are Vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), midkine and thymidine phosphorylase [17]. Prognostic impact has been reported for VEGF, FGF and HGF. A recent meta-analysis on data of 29 studies and 2345 patients reexamined the role of VEGF in esophageal cancer. Esophageal SCC patients with elevated levels had a 1.82-fold greater risk of death, but no association with poor survival in AEG was shown [18]. Several studies indicate that HGF is overexpressed in SCC tissue specimen and serum levels are associated with survival and clinicopathological parameters such as distant metastases [19-21]. Another angiogenic factor associated with poor survival is FGF [22,23].
The aim of this retrospective exploratory study was to investigate the complex angiogenic cytokine expression both in tissue and serum at the time of resection in patients with neoadjuvantly treated esophageal cancer. To elucidate the complexity of this network a commercial multiplex assay for 9 angiogenic factors was utilized. Different cytokine profiles linked to therapeutic outcome could help establish anti-angiogenic targets that combined with chemo(radio)therapy could improve response to therapy in a defined subgroup of patients.
Methods
Patient data
78 patients with esophageal squamous cell carcinoma (n = 34) and adenocarcinoma of the gastroesophageal junction (AEG I/II) (n = 44) were included in the study. These patients were treated in the Department of Surgery, University of Heidelberg, Germany between 2007 and 2012. All patients underwent neoadjuvant chemo(radio)therapy followed by resection or explorative operation according to the current guidelines. Patients received neoadjuvant therapy as mostly on an out-patient basis from their oncologist. Therapies regimens were decided by the oncologist or after presentation at an interdisciplinary tumor board.
Patients with adenocarcinoma (44 pts) of the gastroesophageal junction were treated with either Epirubicin containing regimens (EOX, ECF) (29/44; 65,9%) or alternatively with a 5-FU/platinum based regime (FLOT/FLO/FOLFOX/PLF) (9/44; 20,5%). 5 patients (5/44; 11,4%) with AEG received chemoradiotherapy, 1 patient received a Taxol based chemotherapy. Chemoradiotherapy combined with Cisplatin and 5-Fluoruracil was given to all patients (34 pts) with esophageal squamous cell carcinoma (34/34; 100%). Details on neoadjuvant treatment regimens are shown in Additional file 1: Table S1A.
As preoperative staging all patients received a CT scan and an endoscopy. Patients with a decrease of tumor mass in endoscopy and a >50% decline in wall thickness seen in the CT and/or endoscopic ultrasound were defined as clinical responders [24].
Most patients underwent abdominothoracic en bloc esophagectomy including a 2-field-lymphadenectomy with anastomosis either in the anterior or in the posterior mediastinum or a transhiatal esophagectomy with anastomosis in the neck. Patients with AEG II received a transhiatal gastrectomy with D2-lymphadenectomy. Operative procedures are shown in Additional file 1: Table S1B. 14 Patients received adjuvant therapy. 12 received adjuvant chemotherapy, 1 additive chemotherapy and 1 palliative chemoradiotherapy.
All patients gave written informed consent. The study protocol was approved by the Ethical Committee of the University of Heidelberg.
Follow up
Most patients received follow-up examinations in the National Center for Tumor Diseases, Heidelberg. Patient follow-up was performed quarterly in the first year, every six months in the second and third year and annually in the fourth or fifth year after resection. In case of follow-up by an oncologist, patients were contacted by phone and the oncologist was contacted by mail. Median follow-up of the surviving patients was 40.62 months, one patient was lost to follow-up.
Histopathology
Pathological specimens were analysed in the Department of Pathology of the University Hospital Heidelberg. Histopathological staging comprised TNM classification, R-category and tumor regression rate (TRG). All patients were re-classified according to the 7th edition of the TNM staging.
To specify TRG Becker regression score was used:
1a—complete regression (no residual tumor)
1b—subtotal regression (<10% residual tumor)
2—minor regression (10–50% residual tumor)
3—no regression (>50% residual tumor).
We classified patients with regression grades 1a and 1b as histopathological responders, those with grades 2 and 3 as non-responders. In addition we used 50% residual tumor as another cut-off point for response, to take the limited number of patients into account.
Blood and tissue sampling and preparation
One day before tumor resection blood was collected in serum tubes from a central venous catheter or by peripheral venous sampling. Before blood was taken from the central venous catheter the first 5 ml of the drawn blood were discarded to avoid dilution with blocking saline. To extract the serum the tubes were centrifugated at 2.500 g for 10 minutes. Serum was then stored at −80°C until analysis. Before analysis serum was diluted 1:4 with a sample diluent.
The tissue specimens were collected directly after tumor resection and then stored at −80°C. Sections of 10 μm were cut with a cryotome. Sections were then transferred into a lysis buffer. The concentration of the lysed tissue samples was adjusted to 600 μg/ml.
Cytokine detection
We detected serum and tissue concentrations of Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1), Vascular Endothelial Growth Factor (VEGF), Leptin, Angiopoietin-2 (Ang-2), Follistatin, Granulocyte-Colony Stimulating Factor (GCSF), Hepatocyte Growth Factor (HGF), Platelet-Derived Growth Factor (PDGF) and Interleukin-8 (IL-8). Cytokine levels were measured using the BioRad Bio-Plex Human Angiogenesis Assay (Bio-Rad Laboratories, Inc., Hercules, CA 94547, USA) and Luminex two-laser array reader (Bioplex200). Bioplex Manager 6.1. (Bio-Rad Laboratories, Inc., Hercules, CA 94547, USA) was used to acquire standard curves and concentrations.
Statistical analysis
Continuous variables are expressed as median and inter quartile ranges (IQR) with additional 95% confidence interval. To compare differences in medians we used the Mann–Whitney-U test or Kruskal-Wallis H-Test for multiple comparisons between the groups. Additional multiple ANOVA (MANOVA) testing was performed for multiple hypothesis testing in multiple comparisons. Categorical data is presented in absolute and relative frequencies. Comparison was performed by the Chi-square-test. To compare angiogenic cytokine levels we used the median as a cut-off.
We measured overall survival from point of diagnosis until death. The Kaplan Meier Method was performed for survival analysis, for differences in survival time we used the log-rank test. Results for overall survival were confirmed with multi-variate Cox regression analysis. Receiver-operating characteristic (ROC) curves and area under the ROC curve (AUC) were used to assess the diagnostic value of Follistatin and HGF. A ROC curve was created to select the Youden’s index (Youden’s index = sensitivity + specificity − 1), and the highest sensitivity and specificity were selected as the cutoff values. Statistical significance was taken as a p-value of <0.05 (two-tailed). All statistical analyses were done using SPSS software version 20.0 (SPSS, Inc., Chicago, Illinois, USA).
Results
Patients’ pretherapeutic and postoperative clinicopathological characteristics are displayed in Tables 1 and 2. We included 78 patients, 34 (43.6%) with esophageal squamous cell carcinoma and 44 (56.4%) with adenocarcinoma of the gastroesophageal junction (AEG I/II).
Prognostic clinicopathological factors
As relevant prognostic factors in all patients we found the pN-category (p = 0.001). Additionally tumor regression grade (TRG) was found to be a significant prognostic factor in all patients (p = 0.052) and in adenocarcinoma of the gastroesophageal junction (p = 0.048). Clinical and histopathological reponse and 3-year survival rates of SCC and AEG are displayed in Additional file 2: Table S2.
Correlation of angiogenic factors with clinicopathological factors
Angiopoietin-2 and Follistatin protein levels within the tumor tissue were associated with tumor type (p = 0.022 and p = 0.001). Patients with SCC had higher levels of Angiopoietin-2 and Follistatin levels than patients with AEG (Table 3). Circulating angiogenic factors did not correlate with other clinicopathological factors (Table 4).
Angiogenic cytokines and response to chemotherapy
As shown in Tables 3 and 4 angiogenic cytokine levels either circulating or within the tissue were not associated with clinical response or TRG for all patients as well as in the AEG and SCC subgroups (Additional file 3: Table S3). Defining histopathological non-responding patients as having more than 50% remaining tumor cell mass in histopathological analysis, histopathological non-responders showed lower tissue levels of Follistatin than patients with more than 50% remaining tumor cells (p = 0.015).
Prognostic impact of angiogenic cytokines
To evaluate the prognostic impact of the angiogenic cytokines, Kaplan-Maier survival analysis were performed for each angiogenic factor with the median as cut-off. In the patients serum no angiogenic cytokine was found to be associated with survival time. In the tissue samples HGF and Follistatin levels were associated with patients’ prognosis (p = 0.037; p = 0.036) (Figures 1A and B). Median survival of patients with lower levels of HGF was not reached at time of analysis, while median survival of patients with higher levels was 20.3 ± 4,3 (11,7 – 28,8 95%CI) months. Median survival of patients with lower levels of Follistatin was 36,7 months (95% CI not reached), compared to 16,0 ± 2,7 (10,6 – 21,4 95% CI) months for patients with higher levels. The median survival and the significant cytokines for all patients are presented in Tables 5 and 6. When performing multivariate cox regression analysis including all angiogenic factors in tissue, Follitstatin levels remained associated to overall survival (p = 0.04).
Prognostic cytokines in the tissue of esophageal cancer patients. Kaplan-Maier plots for overall survival of esophageal cancer patients according to A) tissue protein levels of HGF (smaller and higher then median of 5417,1 pg/ml) and B) tissue protein levels of follistatin (smaller and higher then median of 557,7 pg/ml) (n = 58; p < 0.05).
In AEG tumor tissues alone HGF and Leptin were found to be associated with patients’ survival (p = 0.028 and p = 0.034) (Additional file 4: Tables S4 A and B). In SCC alone no angiogenic cytokine in the tumor tissue reached statistical significance. A lower Angiopoietin-2/VEGF-Ratio in the serum was associated with longer survival in patients with SCC (p = 0.032) (Additional file 4: Tables S4 C and D).
Receiver Operating Characteristics (ROC) curves
We performed Receiver Operating Characteristics for the statistically significant cytokines to determine possible cut-offs that are more appropriate than the median. The calculated optimal cut-offs are similar to the ones using the median as cut-off. Receiver operating charateristics with the optimal cut-offs, sensitivity and specificity are shown in Table 7.
Discussion
In this study a panel of angiogenic factors in neoadjuvantly treated patients with esophageal cancer (AEGI/II and SCC) that underwent tumor resection in the Department of Surgery at the University of Heidelberg was analyzed in the tumor tissue and serum. Of the angiogenic factors VEGF, Angiopoietin-2, PECAM-1, Follistatin, Leptin, G-CSF, HGF, PDGF-BB and IL-8, two angiogenic factors (HGF and Follistatin) are associated with esophageal cancer patients’ prognosis in posttherapeutic tumor tissue. Expression levels of most angiogenic factors in AEG and SCC were similar, biological differences of these two entities of esophageal cancer with respect to angiogenesis are indicated by the different expression of Angiopoietin-2 and Follistatin. No association with response to neoadjuvant therapy could be observed.
Results of this study are of current importance as antiangiogenic therapy has found its way into clinical therapy regimens. As a result the complex interactions in the tumor microenvironment and the role of angiogenesis within it receive more and more interest. It has yet to be determined if the angiogenic microenvironment influences neoadjuvant therapy response in esophageal cancer patients. Currently reliable clinical or molecular markers to evaluate response are still lacking, even though metabolic response prediction has recently been evaluated as a method [25]. Response prediction is becoming of exceeding interest especially in regard to the chosen therapeutical regimen considering that response rates to classical chemo(radio)therapeutical regimens do mostly not exceed 50% [7]. This analysis of the angiogenic phenotype within the tumor and its tumor microenvironment and its relation to therapy response adds further insight into this problem. As key hallmark of cancer, angiogenesis is activated early in tumorigenesis and contributes to tumor progression and metastasis by influencing and shaping the tumor microenvironment [11]. The “angiogenic switch” occurs when the balance between pro- and anti-angiogenic factors is shifted towards the pro-angiogenic side, resulting in increased angiogenesis and tumor growth [26]. In esophageal cancers angiogenesis has been described to be involved in tumor growth and metastasis [17] and the prototypic angiogenic factor VEGF is often associated with poor survival. Other pro-angiogenic factors such as HGF, FGF and IL-8 have been reported to play a role in esophageal cancer [18,27]. However most studies were limited to one or two investigated factors, curbing conclusions about the complex network of different angiogenic factors. A strength of our study is the analysis of a comprehensive angiogenic factor panel in both blood and corresponding tumor tissue in most of the patients. Furthermore all included patients underwent neoadjuvant treatment.
Limitations of our study are the limited number of patients and the retrospective design, with all its known and accepted problems. Furthermore, all esophageal cancer entities are analyzed together, which might be criticized by some groups, despite the fact that the UICC classification [28] and landmark studies like the CROSS Study [5,29] also combine them. To address this issue we additionally provided subgroup analysis. As further limitations both tumor and serum samples was available in only 70% of the patients and different preoperative treatment regimens were applied, although both are accepted standards in esophageal cancer [4-6,30,31].
We found that in the tumor tissue Follistatin and Angiopoietin-2 are differentially expressed between the two tumor types with significantly higher median tissue levels of the two angiogenic factors in SCC patients when compared to AEG patients. These general differences in expression support the hypothesis that AEG and SCC represent cancer types with at least some biological differences in angiogenesis. Similarly differences in etiology, epidemiology and tumor biology between AEG and SCC have been reported before [2], but both tumor entities are classified identically within UICC 2010 [28] and analyzed together in most studies [5].
Hepatocyte Growth Factor (HGF) and Follistatin in the tumor tissue were associated with patient survival. Using the median as cut-off, high HGF and Follistatin expression in the tumor tissue were associated with poor patients’ prognosis. ROC analysis aimed to evaluate if a more appropriate cut-off value then the median should be used. However the calculated “optimal” cut-off values were actually close to the used median and support its use as cut-off value in this study. The low value of the AUC is likely related to the small sample size for this calculation, however it underlines the need of additional larger studies in the future.
HGF and its receptor c-Met play an important role in the development of various cancer types. HGF, also known as scatter factor is a growth factor directed to epithelial cells that is active in embryogenesis and mediates defense to tissue damage in adults. HGF binds c-Met, a tyrosine kinase receptor as exclusive ligand induces the activation of oncogenic pathways, angiogenesis and scattering of cells, leading to metastasis [32]. Supporting our findings concerning HGF, Tuynman et al. found Met expression to be an independent prognostic risk factor in esophageal adenocarcinoma [33]. Furthermore, it has been reported that higher HGF levels in serum and tissue are associated with tumor progression and poor survival in esophageal squamous cell carcinoma [19,20]. Ren et al. found pretherapeutic HGF levels as an independent prognostic factor [19]. In our data we did not find a correlation of HGF in the patients’ sera with survival. This could be due to the point of time when the blood sampling took place. Neoadjuvant therapy may influence HGF serum levels in a different way than in the tumor tissue.
Follistatin tissue levels were significantly associated with patients’ prognosis. Elevated levels of Follistatin as an antagonist of the TGFβ superfamily member Activin A have been reported in solid tumors [34-37], however no data on esophageal cancer have been published so far. Interestingly tissue levels of Follistatin differed between non-responders and responders, when defining responders as having less than 50% remaining tumor cell mass (p = 0.015). The definitions of histopathological response after chemotherapy or radiochemotherapy are heterogenous varying from a pCR up to 50% residual tumor and would need clear definitions to make study results comparable.
We investigated angiogenic cytokine levels with regard to patients’ clinical and histopathological response and prognosis. Considering the fact that all patients received neoadjuvant chemo(radio)therapy it is interesting that we found no association between circulating angiogenic cytokines and patients survival or response. This could be related to the point in time of posttherapeutic measurement of the factors. Neoadjuvant therapy might influence circulating angiogenic cytokine levels and thus change levels after chemo(radio)therapy. Most studies investigated angiogenic factors in previously untreated patients [19,38-41].
Based on these interesting obtained results we would recommend a prospective validation of this data in a patient cohort from multiple centers. If the results are confirmed a large multicenter trial with a central bio-banking should be performed. This should be included within the next trials on neoadjuvant or perioperative chemo(radio)therapy. It would be favorable to obtain blood samples in a standardized manner, pre-therapeutic, during therapy, before surgery and during the follow up. Tumor tissue could also be obtained during initial biopsy and from the pathological sample. With this information it will be possible to provide a more complete picture of the tumor biology. The aim will be to identify deregulated targetable pathways as i.e. the HGF/met pathway.
In summary, further studies will be necessary to investigate the impact of neoadjuvant therapy on tumor microenvironment and circulating angiogenic cytokines. Pretherapeutic and preoperative blood sampling could elucidate the change of angiogenic factor levels over time of therapy. As the limited number of patients may have influenced the results of this study, investigations in larger patient cohorts could confirm our findings and evaluate angiogenic cytokine levels as pretherapeutic diagnostic factors – helping the physician to choose the right therapy.
Conclusion
Angiogenic cytokines seem to play an important role in patients with esophageal cancer. In this study two angiogenic factors (HGF and Follistatin) in tumor tissue after neoadjuvant therapy were associated with esophageal cancer patients’ prognosis. HGF levels seem to be important with regard to tumor development in esophageal cancer. Different expression of angiogenic cytokines in AEG and SCC subgroups confirm the assumption that the two represent different entities with respect to angiogenesis. The lack of association with response to therapy could be explained by the fact that samples were collected at a preoperative and not pretherapeutic point. Results are promising and should be pursued prospectively in a pre- and posttherapeutic state. Further studies with larger number of patients seem to be necessary.
References
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12.
Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17(1):38–44.
Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52.
Cunningham D, Allum W, Stenning S, Thompson J, Van de Velde C, Nicolson M. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
van Hagen PHM, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BPRD, Nieuwenhuijzen GA, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–92.
Blank S, Stange A, Sisic L, Roth W, Grenacher L, Sterzing F, et al. Preoperative therapy of esophagogastric cancer: the problem of nonresponding patients. Langenbecks Arch Surg. 2012;398(2):211–20.
Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–25.
Brucher BL, Stein HJ, Zimmermann F, Werner M, Sarbia M, Busch R, et al. Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol. 2004;30(9):963–71.
Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–54.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64.
Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1(1):12–25.
Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61(3):253–70.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Okines AF, Reynolds AR, Cunningham D. Targeting angiogenesis in esophagogastric adenocarcinoma. Oncologist. 2011;16(6):844–58.
Oshima Y, Yajima S, Yamazaki K, Matsushita K, Tagawa M, Shimada H. Angiogenesis-related factors are molecular targets for diagnosis and treatment of patients with esophageal carcinoma. Ann Thorac Cardiovasc Surg. 2010;16(6):389–93.
Chen M, Cai E, Huang J, Yu P, Li K. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1126–34.
Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung L, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11(17):6190–7.
Takada N, Yano Y, Matsuda T, Otani S, Osugi H, Higashino M, et al. Expression of immunoreactive human hepatocyte growth factor in human esophageal squamous cell carcinomas. Cancer Lett. 1995;97(2):145–8.
Xu Z, Wang S, Wu M, Zeng W, Wang X, Dong Z. TGFbeta1 and HGF protein secretion by esophageal squamous epithelial cells and stromal fibroblasts in oesophageal carcinogenesis. Oncol Lett. 2013;6(2):401–6.
Han B, Liu J, Ma MJ, Zhao L. Clinicopathological significance of heparanase and basic fibroblast growth factor expression in human esophageal cancer. World J Gastroenterol. 2005;11(14):2188–92.
Sugiura K, Ozawa S, Kitagawa Y, Ueda M, Kitajima M. Co-expression of aFGF and FGFR-1 is predictive of a poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep. 2007;17(3):557–64.
Heger U, Bader F, Lordick F, Burian M, Langer R, Dobritz M, et al. Interim endoscopy results during neoadjuvant therapy for gastric cancer correlate with histopathological response and prognosis. Gastric Cancer. 2014;17(3):478–88.
Ott K, Herrmann K, Schuster T, Langer R, Becker K, Wieder HA, et al. Molecular imaging of proliferation and glucose utilization: utility for monitoring response and prognosis after neoadjuvant therapy in locally advanced gastric cancer. Ann Surg Oncol. 2011;18(12):3316–23.
Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–37.
Diakowska D. Cytokines association with clinical and pathological changes in esophageal squamous cell carcinoma. Dis Markers. 2013;35(6):883–93.
Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116(16):3763–73.
Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32(5):385–91.
Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19(11):1882–7.
Boige V, Pignon J, Saint-Aubert B. Final results of a randomized trial comparing preoperative 5-Fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol (supplement). 2007;25(18 Suppl):15123.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25.
Tuynman JB, Lagarde SM, Ten Kate FJ, Richel DJ, van Lanschot JJ. Met expression is an independent prognostic risk factor in patients with oesophageal adenocarcinoma. Br J Cancer. 2008;98(6):1102–8.
Yuen MF, Norris S, Evans LW, Langley PG, Hughes RD. Transforming growth factor-beta 1, activin and follistatin in patients with hepatocellular carcinoma and patients with alcoholic cirrhosis. Scand J Gastroenterol. 2002;37(2):233–8.
Ren P, Chen FF, Liu HY, Cui XL, Sun Y, Guan JL, et al. High serum levels of follistatin in patients with ovarian cancer. J Int Med Res. 2012;40(3):877–86.
Tumminello FM, Badalamenti G, Fulfaro F, Incorvaia L, Crescimanno M, Flandina C, et al. Serum follistatin in patients with prostate cancer metastatic to the bone. Clin Exp Metastasis. 2010;27(8):549–55.
Blank S, Deck C, Dreikhausen L, Weichert W, Giese N, Falk C, et al. Angiogenic and growth factors in gastric cancer. J Surg Res. 2014 Nov 26. [Epub ahead of print].
Chen MF, Chen PT, Lu MS, Lin PY, Chen WC, Lee KD. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer. 2013;12:26.
Shimada H, Takeda A, Nabeya Y, Okazumi SI, Matsubara H, Funami Y, et al. Clinical significance of serum vascular endothelial growth factor in esophageal squamous cell carcinoma. Cancer. 2001;92(3):663–9.
Mobius C, Freire J, Becker I, Feith M, Brucher BL, Hennig M, et al. VEGF-C expression in squamous cell carcinoma and adenocarcinoma of the esophagus. World J Surg. 2007;31(9):1768–72. discussion 1773–1764.
Diakowska D, Krzystek-Korpacka M, Markocka-Maczka K, Diakowski W, Matusiewicz M, Grabowski K. Circulating leptin and inflammatory response in esophageal cancer, esophageal cancer-related cachexia-anorexia syndrome (CAS) and non-malignant CAS of the alimentary tract. Cytokine. 2010;51(2):132–7.
Acknowledgements
The authors thank the Heidelberg Surgery Foundation for the funding of this project. This study was approved by the local ethics commission. We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LK, SB, LS, NG, CF, KO and TS contributed to the study design. Data acquisition was carried out by LD, SB, LS, UH, WW, DJ, LG, CF, KO and TS. LD, SB, TB, SF, KO and TS contributed to data analysis. LD, KO and TS drafted the manuscript and SB, LS, UH, WW, DJ, TB, NG, LG and CF reviewed the manuscript. All authors read and approved the final version of the manuscript.
Additional files
Additional file 1: Table S1.
A) neoadjuvant treatment regimens. B) operative procedures.
Additional file 2: Table S2.
Response to neoadjuvant therapy.
Additional file 3: Table S3.
Association of angiogenic cytokines with clinical response and tumor regression grade (TRG) in AC and SCC.
Additional file 4: Table S4.
A) Prognostic significance of circulating angiogenic factor levels in AEG I/II. B) Prognostic significance of tissue angiogenic factor levels in AEG I/II. C) Prognostic significance of circulating angiogenic factor levels in SCC. D) Prognostic significance of tissue angiogenic factor levels in SCC.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dreikhausen, L., Blank, S., Sisic, L. et al. Association of angiogenic factors with prognosis in esophageal cancer. BMC Cancer 15, 121 (2015). https://doi.org/10.1186/s12885-015-1120-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1120-5