Abstract
Purpose
To assess the pharmacokinetics, pharmacodynamics and tolerability of different doses of octreotide and pasireotide (subcutaneous [sc] and long-acting release [LAR]) when co-administered in healthy volunteers.
Methods
This was an exploratory, Phase I, single-centre study. Healthy adults were enrolled in a staggered approach into seven cohorts to receive octreotide and pasireotide (sc and LAR formulations), alone or in combination. Plasma drug concentrations, growth hormone (GH), insulin-like growth factor I (IGF-I), and plasma glucose were assessed at baseline, immediately after sc treatment, and 21 and 28 days after LAR treatment.
Results
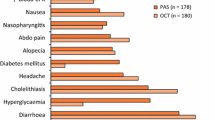
Of 88 enrolled subjects, 52 and 82 participated in sc and LAR dosing phases, respectively. There were no relevant pharmacokinetic interactions between octreotide and pasireotide. In combination, pasireotide sc (150 µg) and octreotide sc (100/300 µg) resulted in numerically greater reductions in insulin levels and a higher incidence of AEs than either single agent; the rapid (within 1 h) increase in plasma glucose after pasireotide was delayed with combination treatment. Octreotide sc and pasireotide sc, alone or in combination, reduced IGF-I levels and led to undetectable GH levels in most subjects. During the LAR phase, addition of a low dose of pasireotide (5 mg) to a standard dose of octreotide (20 mg) resulted in an ~2-fold reduction in median IGF-I versus octreotide 20 mg 21 days post-dose; this effect was numerically greater than seen for pasireotide 20 mg alone. Peak plasma glucose was substantially lower after LAR than sc dosing. Interestingly, glucose levels were also numerically lower in the pasireotide 5 mg plus octreotide 20 mg group than for 20 mg of octreotide or pasireotide alone. AEs were less frequent after LAR than sc dosing.
Conclusions
Combined low doses of pasireotide LAR (5 mg) and octreotide LAR (10–30 mg) provided greater suppression of IGF-I than either single agent and did not increase blood glucose or incidence of AEs versus either agent alone.


Similar content being viewed by others
Data availability
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. This trial data availability is in accordance with the criteria and process described on www.clinicalstudydatarequest.com.
References
P.U. Freda, Somatostatin analogs in acromegaly. J. Clin. Endocrinol. Metab. 87(7), 3013–3018 (2002). https://doi.org/10.1210/jcem.87.7.8665
Y.C. Patel, Somatostatin and its receptor family. Front. Neuroendocrinol. 20(3), 157–198 (1999). https://doi.org/10.1006/frne.1999.0183
H.A. Schmid, J. Brueggen, Effects of somatostatin analogs on glucose homeostasis in rats. J. Endocrinol. 212(1), 49–60 (2012). https://doi.org/10.1530/JOE-11-0224
J. van der Hoek, M. Waaijers, P.M. van Koetsveld, D. Sprij-Mooij, R.A. Feelders, H.A. Schmid, P. Schoeffter, D. Hoyer, D. Cervia, J.E. Taylor, M.D. Culler, S.W. Lamberts, L.J. Hofland, Distinct functional properties of native somatostatin receptor subtype 5 compared with subtype 2 in the regulation of ACTH release by corticotroph tumor cells. Am. J. Physiol. Endocrinol. Metab. 289(2), E278–E287 (2005). https://doi.org/10.1152/ajpendo.00004.2005
K. Oberg, Management of neuroendocrine tumours. Ann. Oncol. 15(Suppl 4), iv293–iv298 (2004). https://doi.org/10.1093/annonc/mdh942
L. Katznelson, E.R. Laws Jr, S. Melmed, M.E. Molitch, M.H. Murad, A. Utz, J.A.H. Wass, Acromegaly: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(11), 3933–3951 (2014). https://doi.org/10.1210/jc.2014-2700
S. Melmed, M.D. Bronstein, P. Chanson, A. Klibanski, F.F. Casanueva, J.A.H. Wass, C.J. Strasburger, A. Luger, D.R. Clemmons, A. Giustina, A consensus statement on acromegaly therapeutic outcomes. Nat. Rev. Endocrinol. 14(9), 552–561 (2018). https://doi.org/10.1038/s41574-018-0058-5
C. Bruns, I. Lewis, U. Briner, G. Meno-Tetang, G. Weckbecker, SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 146(5), 707–716 (2002). https://doi.org/10.1530/eje.0.1460707
M.R. Gadelha, M.D. Bronstein, T. Brue, M. Coculescu, M. Fleseriu, M. Guitelman, V. Pronin, G. Raverot, I. Shimon, K.K. Lievre, J. Fleck, M. Aout, A.M. Pedroncelli, A. Colao, Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2(11), 875–884 (2014). https://doi.org/10.1016/s2213-8587(14)70169-x
A. Colao, M.D. Bronstein, P. Freda, F. Gu, C.C. Shen, M. Gadelha, M. Fleseriu, A.J. van der Lely, A.J. Farrall, K. Hermosillo Resendiz, M. Ruffin, Y. Chen, M. Sheppard, C.S.G. Pasireotide, Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J. Clin. Endocrinol. Metab. 99(3), 791–799 (2014). https://doi.org/10.1210/jc.2013-2480
A. Colao, S. Petersenn, J. Newell-Price, J.W. Findling, F. Gu, M. Maldonado, U. Schoenherr, Dipl.-Biol., D. Mills, L.R. Salgado, B.M.K. Biller, A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl J. Med. 366(10), 914–924 (2012). https://doi.org/10.1056/NEJMoa1105743
A. Lacroix, F. Gu, W. Gallardo, R. Pivonello, Y. Yu, P. Witek, M. Boscaro, R. Salvatori, M. Yamada, L. Tauchmanova, M. Roughton, S. Ravichandran, S. Petersenn, B.M.K. Biller, J. Newell-Price, G. Arnaldi, H.S. Asha, T. Bandgar, A. Barkan, H. Biering, M. Bex, M. Bolanowski, M.D. Bronstein, T. Brue, D. Bruera, F. Cavagnini, A. Comlekci, C. De Block, T. Delibasi, C. Fajardo-Montañana, R.A. Feelders, M. Fleseriu, M.R. Gadelha, E.B. Geer, A. Heaney, G. Houde, A. Ichihara, S.A. Imran, A. Ioachimescu, P. Kadioglu, Y. Li, P. Loli, M. Nishiyama, L. Rozhinskaya, M. Ruchala, Y. Saitoh, C. Schöfl, J. Schopohl, A. Shimatsu, C. Shimizu, T. Snabboon, P. Snyder, N. Suzaki, A. Tabarin, Y. Takahashi, S.T. Britto, G. T’Sjoen, M.-C. Vantyghem, B. Velkeniers, S. Webb, S. Yamada, Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 6(1), 17–26 (2018). https://doi.org/10.1016/s2213-8587(17)30326-1
U. Kumar, R. Sasi, S. Suresh, A. Patel, M. Thangaraju, P. Metrakos, S.C. Patel, Y.C. Patel, Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48(1), 77–85 (1999). https://doi.org/10.2337/diabetes.48.1.77
R.R. Henry, T.P. Ciaraldi, D. Armstrong, P. Burke, M. Ligueros-Saylan, S. Mudaliar, Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J. Clin. Endocrinol. Metab. 98(8), 3446–3453 (2013). https://doi.org/10.1210/jc.2013-1771
S. Chiloiro, A. Giampietro, F. Visconti, L. Rossi, F. Donfrancesco, C.M. Fleseriu, F. Mirra, A. Pontecorvi, A. Giustina, M. Fleseriu, L. De Marinis, A. Bianchi, Glucose metabolism outcomes in acromegaly patients on treatment with pasireotide-LAR or pasireotide-LAR plus pegvisomant. Endocrine 73(3), 658–666 (2021). https://doi.org/10.1007/s12020-021-02711-3
M.R. Gadelha, F. Gu, M.D. Bronstein, T.C. Brue, M. Fleseriu, I. Shimon, A.J. van der Lely, S. Ravichandran, A. Kandra, A.M. Pedroncelli, A.A.L. Colao, Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr. Connect. 9(12), 1178–1190 (2020). https://doi.org/10.1530/EC-20-0361
A. Colao, R.S. Auriemma, R. Pivonello, L. Kasuki, M.R. Gadelha, Interpreting biochemical control response rates with first-generation somatostatin analogues in acromegaly. Pituitary 19(3), 235–247 (2016). https://doi.org/10.1007/s11102-015-0684-z
M. Kugita, K. Nishii, T. Yamaguchi, A. Suzuki, Y. Yuzawa, S. Horie, E. Higashihara, S. Nagao, Beneficial effect of combined treatment with octreotide and pasireotide in PCK rats, an orthologous model of human autosomal recessive polycystic kidney disease. PLoS ONE 12(5), e0177934 (2017). https://doi.org/10.1371/journal.pone.0177934
D.S. Wald, M. Law, J.K. Morris, J.P. Bestwick, N.J. Wald, Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am. J. Med. 122(3), 290–300 (2009). https://doi.org/10.1016/j.amjmed.2008.09.038
L. Kuritzky, G.P. Samraj, Enhanced glycemic control with combination therapy for type 2 diabetes in primary care. Diabetes Ther. 2(3), 162–177 (2011). https://doi.org/10.1007/s13300-011-0006-z
F. Tiberg, J. Roberts, C. Cervin, M. Johnsson, S. Sarp, A.P. Tripathi, M. Linden, Octreotide s.c. depot provides sustained octreotide bioavailability and similar IGF-1 suppression to octreotide LAR in healthy volunteers. Br. J. Clin. Pharmacol. 80(3), 460–472 (2015). https://doi.org/10.1111/bcp.12698
F. Tiberg, S. Glantz, K. Strangarden, C. Darstein, J. Eisinger, L. Tauchmanova, A. Breitschaft, A first-in-human pharmacokinetic, safety, and tolerability study of pasireotide subcutaneous depot. Endocrine Abstracts 56, P856 (2018)
J. Amaru, F. Barbieri, M. Arvigo, A. Solari, A. Bajetto, F. Nista, C. Campana, G. Gaggero, A. Prior, D. Criminelli Rossi, G. Zona, D. Ferone, T. Florio, F. Gatto, Octreotide and pasireotide combination treatment in somatotroph tumor cells: predominant role of SST2 in mediating ligand effects. Cancers 13(8) (2021). https://doi.org/10.3390/cancers13081816
R. Attanasio, A. Mainolfi, F. Grimaldi, R. Cozzi, M. Montini, C. Carzaniga, S. Grottoli, L. Cortesi, M. Albizzi, R.M. Testa, L. Fatti, D. De Giorgio, C. Scaroni, F. Cavagnini, P. Loli, G. Pagani, E. Ghigo, Somatostatin analogs and gallstones: a retrospective survey on a large series of acromegalic patients. J. Endocrinol. Investig. 31(8), 704–710 (2008). https://doi.org/10.1007/BF03346419
Acknowledgements
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Robert Jenn Ph.D., Mudskipper Business Ltd, for medical writing assistance with this paper. We also thank the site investigators, study coordinators and subjects who participated in the study.
Funding
This study was sponsored by Novartis Pharma AG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.T. is a former employee of Novartis. A.B. is an employee of Parexel International GmbH; Parexel International GmbH was paid by Novartis Pharma AG for assistance in conducting the study. G.H., K.T.H., S.C., C.D., M.P., E.D., G.G. and H.A.S. are employees of Novartis. A.M.P. is a former employee of Novartis and a current employee of Recordati.
Ethical approval
The protocol and all amendments were reviewed and approved by the Berlin State Ethics Committee (Turmstraße 21, Haus A, Berlin 10559, Germany). The study was conducted according to the ICH E6 Guideline for Good Clinical Practice and the ethical principles of the Declaration of Helsinki.
Consent to participate
All subjects provided written consent to participate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Tauchmanova, L., Breitschaft, A., Holder, G. et al. Combination of pasireotide and octreotide: effects on GH and IGF-I secretion and glucose metabolism in healthy volunteers. Endocrine 75, 537–548 (2022). https://doi.org/10.1007/s12020-021-02908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02908-6