Abstract
Purpose
Bone may regulate glucose homeostasis via uncarboxylated bioactive osteocalcin (ucOCN). This study explored whether changes in ucOCN and bone remodeling are associated with change in glucose homeostasis after biliopancreatic diversion (BPD).
Methods
In this secondary exploratory analysis of a 1-year prospective observational study, 16 participants (11 men/5 women; 69% with type 2 diabetes; mean BMI 49.4 kg/m2) were assessed before, 3 days, 3 months and 12 months after BPD. Changes in plasma ucOCN and bone markers (C-terminal telopeptide (CTX), total osteocalcin (OCN)) were correlated with changes in insulin resistance or sensitivity indices (HOMA-IR; adipose tissue insulin resistance index (ADIPO-IR) and insulin sensitivity index (SI) from the hyperinsulinemic-euglycemic clamp), insulin secretion rate (ISR) from the hyperglycemic clamp, and disposition index (DI: SI × ISR) using Spearman correlations before and after adjustment for weight loss.
Results
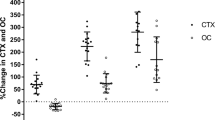
ucOCN was unchanged at 3 days but increased dramatically at 3 months (+257%) and 12 months (+498%). Change in ucOCN correlated significantly with change in CTX at 3 months (r = 0.62, p = 0.015) and 12 months (r = 0.64, p = 0.025) before adjustment for weight loss. It also correlated significantly with change in fasting insulin (r = −0.53, p = 0.035), HOMA-IR (r = −0.54, p = 0.033) and SI (r = 0.52, p = 0.041) at 3 days, and ADIPO-IR (r = −0.69, p = 0.003) and HbA1c (r = −0.69, p = 0.005) at 3 months. Change in OCN did not correlate with any glucose homeostasis indices. Results were similar after adjustment for weight loss.
Conclusion
The increase in ucOCN may be associated with the improvement in insulin resistance after BPD, independently of weight loss. These findings need to be confirmed in larger, less heterogeneous populations.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
G. Mingrone, S. Panunzi, A. De Gaetano, C. Guidone, A. Iaconelli, L. Leccesi, G. Nanni, A. Pomp, M. Castagneto, G. Ghirlanda, F. Rubino, Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 366(17), 1577–1585 (2012). https://doi.org/10.1056/NEJMoa1200111
H. Buchwald, R. Estok, K. Fahrbach, D. Banel, M.D. Jensen, W.J. Pories, J.P. Bantle, I. Sledge, Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am. J. Med. 122(3), 248–256.e245 (2009). https://doi.org/10.1016/j.amjmed.2008.09.041
H. Buchwald, Y. Avidor, E. Braunwald, M.D. Jensen, W. Pories, K. Fahrbach, K. Schoelles, Bariatric surgery: a systematic review and meta-analysis. JAMA 292(14), 1724–1737 (2004). https://doi.org/10.1001/jama.292.14.1724
C. Guidone, M. Manco, E. Valera-Mora, A. Iaconelli, D. Gniuli, A. Mari, G. Nanni, M. Castagneto, M. Calvani, G. Mingrone, Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 55(7), 2025–2031 (2006). https://doi.org/10.2337/db06-0068
A. Michaud, T. Grenier-Larouche, D. Caron-Dorval, S. Marceau, L. Biertho, S. Simard, D. Richard, A. Tchernof, A.C. Carpentier, Biliopancreatic diversion with duodenal switch leads to better postprandial glucose level and beta cell function than sleeve gastrectomy in individuals with type 2 diabetes very early after surgery. Metab.: Clin. Exp. 74, 10–21 (2017). https://doi.org/10.1016/j.metabol.2017.06.005
T. Grenier-Larouche, A.M. Carreau, A. Geloen, F. Frisch, L. Biertho, S. Marceau, S. Lebel, F.S. Hould, D. Richard, A. Tchernof, A.C. Carpentier, Fatty acid metabolic remodeling during type 2 diabetes remission after bariatric surgery. Diabetes 66(11), 2743–2755 (2017). https://doi.org/10.2337/db17-0414
T. Grenier-Larouche, A.M. Carreau, A.C. Carpentier, Early metabolic improvement after bariatric surgery: the first steps toward remission of type 2 diabetes. Can. J. Diabetes 41(4), 418–425 (2017). https://doi.org/10.1016/j.jcjd.2016.10.013
C.E. Plourde, T. Grenier-Larouche, D. Caron-Dorval, S. Biron, S. Marceau, S. Lebel, L. Biertho, A. Tchernof, D. Richard, A.C. Carpentier, Biliopancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity 22(8), 1838–1846 (2014). https://doi.org/10.1002/oby.20771
C. Koliaki, S. Liatis, C.W. le Roux, A. Kokkinos, The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC Endocr. Disord. 17(1), 50 (2017). https://doi.org/10.1186/s12902-017-0202-6
G. Karsenty, M. Ferron, The contribution of bone to whole-organism physiology. Nature 481(7381), 314–320 (2012). https://doi.org/10.1038/nature10763
P. Mera, M. Ferron, I. Mosialou, Regulation of energy metabolism by bone-derived hormones. Cold Spring Harbor Perspect. Med. (2017). https://doi.org/10.1101/cshperspect.a031666
N.K. Lee, H. Sowa, E. Hinoi, M. Ferron, J.D. Ahn, C. Confavreux, R. Dacquin, P.J. Mee, M.D. McKee, D.Y. Jung, Z. Zhang, J.K. Kim, F. Mauvais-Jarvis, P. Ducy, G. Karsenty, Endocrine regulation of energy metabolism by the skeleton. Cell 130(3), 456–469 (2007). https://doi.org/10.1016/j.cell.2007.05.047
M. Ferron, E. Hinoi, G. Karsenty, P. Ducy, Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl Acad. Sci. USA 105(13), 5266–5270 (2008). https://doi.org/10.1073/pnas.0711119105
J. Lacombe, G. Karsenty, M. Ferron, In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol. Metab. 2(4), 498–504 (2013). https://doi.org/10.1016/j.molmet.2013.08.004
J. Bonneau, G. Ferland, A.D. Karelis, E. Doucet, M. Faraj, R. Rabasa-Lhoret, M. Ferron, Association between osteocalcin gamma-carboxylation and insulin resistance in overweight and obese postmenopausal women. J. Diabetes Complicat. 31(6), 1027–1034 (2017). https://doi.org/10.1016/j.jdiacomp.2017.01.023
U. Razny, D. Fedak, B. Kiec-Wilk, J. Goralska, A. Gruca, A. Zdzienicka, M. Kiec-Klimczak, B. Solnica, A. Hubalewska-Dydejczyk, M. Malczewska-Malec, Carboxylated and undercarboxylated osteocalcin in metabolic complications of human obesity and prediabetes. Diabetes/Metab. Res. Rev. 33(3), (2017). https://doi.org/10.1002/dmrr.2862
Y. Takashi, M. Koga, Y. Matsuzawa, J. Saito, M. Omura, T. Nishikawa, Undercarboxylated osteocalcin can predict insulin secretion ability in type 2 diabetes. J. Diabetes Investig. 8(4), 471–474 (2017). https://doi.org/10.1111/jdi.12601
Q. Guo, H. Li, L. Xu, S. Wu, H. Sun, B. Zhou, Undercarboxylated osteocalcin reverts insulin resistance induced by endoplasmic reticulum stress in human umbilical vein endothelial cells. Sci. Rep. 7(1), 46 (2017). https://doi.org/10.1038/s41598-017-00163-2
D.M. Liu, X.Z. Guo, H.J. Tong, B. Tao, L.H. Sun, H.Y. Zhao, G. Ning, J.M. Liu, Association between osteocalcin and glucose metabolism: a meta-analysis. Osteoporos. Int. 26(12), 2823–2833 (2015). https://doi.org/10.1007/s00198-015-3197-8
A.F. Turcotte, T. Grenier-Larouche, R.V. Ung, D. Simonyan, A.M. Carreau, A.C. Carpentier, F. Mac-Way, L. Michou, A. Tchernof, L. Biertho, S. Lebel, S. Marceau, C. Gagnon, Effects of biliopancreatic diversion on bone turnover markers and association with hormonal factors in patients with severe obesity. Obes. Surg. 29(3), 990–998 (2019). https://doi.org/10.1007/s11695-018-3617-x
J. Lacombe, O. Al Rifai, L. Loter, T. Moran, A.F. Turcotte, T. Grenier-Larouche, A. Tchernof, L. Biertho, A.C. Carpentier, D. Prud'homme, R. Rabasa-Lhoret, G. Karsenty, C. Gagnon, W. Jiang, M. Ferron, Measurement of bioactive osteocalcin in humans using a novel immunoassay reveals association with glucose metabolism and beta-cell function. Am. J. Physiol. Endocrinol. Metab. 318(3), E381–E391 (2020). https://doi.org/10.1152/ajpendo.00321.2019
Standards of Medical Care in Diabetes, Abridged for Primary Care Providers. Clin. Diabetes Publ. Am. Diabetes Assoc. 35(1), 5–26 (2017). https://doi.org/10.2337/cd16-0067
L. Biertho, S. Lebel, S. Marceau, F.S. Hould, F. Julien, S. Biron, Biliopancreatic diversion with duodenal switch: surgical technique and perioperative care. Surg. Clin. N. Am. 96(4), 815–826 (2016). https://doi.org/10.1016/j.suc.2016.03.012
D.R. Matthews, J.P. Hosker, A.S. Rudenski, B.A. Naylor, D.F. Treacher, R.C. Turner, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7), 412–419 (1985)
E. Sondergaard, A.E. Espinosa De Ycaza, M. Morgan-Bathke, M.D. Jensen, How to measure adipose tissue insulin sensitivity. J. Clin. Endocrinol. Metab. 102(4), 1193–1199 (2017). https://doi.org/10.1210/jc.2017-00047
A.C. Carpentier, F. Frisch, D. Cyr, P. Genereux, B.W. Patterson, R. Giguere, J.P. Baillargeon, On the suppression of plasma nonesterified fatty acids by insulin during enhanced intravascular lipolysis in humans. Am. J. Physiol. Endocrinol. Metab. 289(5), E849–E856 (2005). https://doi.org/10.1152/ajpendo.00073.2005
A. Carpentier, S.D. Mittelman, B. Lamarche, R.N. Bergman, A. Giacca, G.F. Lewis, Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am. J. Physiol. 276(6 Pt 1), E1055–E1066 (1999)
R.N. Bergman, M. Ader, K. Huecking, G. Van Citters, Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 51(Suppl 1), S212–S220 (2002)
S.E. Kahn, The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46(1), 3–19 (2003). https://doi.org/10.1007/s00125-002-1009-0
M. Ferron, J. Lacombe, Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch. Biochem. Biophysics 561, 137–146 (2014). https://doi.org/10.1016/j.abb.2014.05.022
A. Diaz-Lopez, M. Bullo, M. Juanola-Falgarona, M.A. Martinez-Gonzalez, R. Estruch, M.I. Covas, F. Aros, J. Salas-Salvado, Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J. Clin. Endocrinol. Metab. 98(11), 4524–4531 (2013). https://doi.org/10.1210/jc.2013-2472
K. Mori, M. Emoto, K. Motoyama, E. Lee, S. Yamada, T. Morioka, Y. Imanishi, T. Shoji, M. Inaba, Undercarboxylated osteocalcin does not correlate with insulin resistance as assessed by euglycemic hyperinsulinemic clamp technique in patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 4(1), 53 (2012). https://doi.org/10.1186/1758-5996-4-53
R. Saucedo, G. Rico, G. Vega, L. Basurto, L. Cordova, R. Galvan, M. Hernandez, E. Puello, A. Zarate, Osteocalcin, under-carboxylated osteocalcin and osteopontin are not associated with gestational diabetes mellitus but are inversely associated with leptin in non-diabetic women. J. Endocrinol. Investig. 38(5), 519–526 (2015). https://doi.org/10.1007/s40618-014-0220-4
M. Ferron, J. Wei, T. Yoshizawa, A. Del Fattore, R.A. DePinho, A. Teti, P. Ducy, G. Karsenty, Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142(2), 296–308 (2010). https://doi.org/10.1016/j.cell.2010.06.003
B. Lee, J. Shao, Adiponectin and energy homeostasis. Rev. Endocr. Metab. Disord. 15(2), 149–156 (2014). https://doi.org/10.1007/s11154-013-9283-3
R. Ye, P.E. Scherer, Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2(3), 133–141 (2013). https://doi.org/10.1016/j.molmet.2013.04.001
X. Wu, H. Motoshima, K. Mahadev, T.J. Stalker, R. Scalia, B.J. Goldstein, Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52(6), 1355–1363 (2003)
J.J. Cao, Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 6, 30 (2011). https://doi.org/10.1186/1749-799x-6-30
B.A. Gower, K. Casazza, Divergent effects of obesity on bone health. J. Clin. Densitom. 16(4), 450–454 (2013). https://doi.org/10.1016/j.jocd.2013.08.010
A.L. Evans, M.A. Paggiosi, R. Eastell, J.S. Walsh, Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J. Bone Miner. Res. 30(5), 920–928 (2015). https://doi.org/10.1002/jbmr.2407
J.S. Walsh, T. Vilaca, Obesity, type 2 diabetes and bone in adults. Calcif. Tissue Int. 100(5), 528–535 (2017). https://doi.org/10.1007/s00223-016-0229-0
J. Wei, M. Ferron, C.J. Clarke, Y.A. Hannun, H. Jiang, W.S. Blaner, G. Karsenty, Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J. Clin. Investig. 124(4), 1–13 (2014). https://doi.org/10.1172/jci72323
C. Gagnon, A.L. Schafer, Bone health after bariatric surgery. JBMR 2(3), 121–133 (2018). https://doi.org/10.1002/jbm4.10048
C. Rousseau, S. Jean, P. Gamache, S. Lebel, F. Mac-Way, L. Biertho, L. Michou, C. Gagnon, Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ (Clin. Res. ed.) 354, i3794 (2016). https://doi.org/10.1136/bmj.i3794
E.W. Yu, M. Wewalka, S.A. Ding, D.C. Simonson, K. Foster, J.J. Holst, A. Vernon, A.B. Goldfine, F. Halperin, Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 101(2), 714–722 (2016). https://doi.org/10.1210/jc.2015-3437
R. Basu, J. Peterson, R. Rizza, S. Khosla, Effects of physiological variations in circulating insulin levels on bone turnover in humans. J. Clin. Endocrinol. Metab. 96(5), 1450–1455 (2011). https://doi.org/10.1210/jc.2010-2877
M.M. Weivoda, C.K. Chew, D.G. Monroe, J.N. Farr, E.J. Atkinson, J.R. Geske, B. Eckhardt, B. Thicke, M. Ruan, A.J. Tweed, L.K. McCready, R.A. Rizza, A. Matveyenko, M. Kassem, T.L. Andersen, A. Vella, M.T. Drake, B.L. Clarke, M.J. Oursler, S. Khosla, Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 11(1), 87 (2020). https://doi.org/10.1038/s41467-019-14003-6
I. Mosialou, S. Shikhel, J.M. Liu, A. Maurizi, N. Luo, Z. He, Y. Huang, H. Zong, R.A. Friedman, J. Barasch, P. Lanzano, L. Deng, R.L. Leibel, M. Rubin, T. Nickolas, W. Chung, L.M. Zeltser, K.W. Williams, J.E. Pessin, S. Kousteni, MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543(7645), 385–390 (2017). https://doi.org/10.1038/nature21697
S. Costantini, C. Conte, Bone health in diabetes and prediabetes. World J. Diabetes 10(8), 421–445 (2019). https://doi.org/10.4239/wjd.v10.i8.421
F. Gossiel, H. Altaher, D.M. Reid, C. Roux, D. Felsenberg, C.C. Gluer, R. Eastell, Bone turnover markers after the menopause: T-score approach. Bone 111, 44–48 (2018). https://doi.org/10.1016/j.bone.2018.03.016
Acknowledgements
The authors would like to thank M. David Simonyan for his help with statistical analyses.
Funding
The Canadian Institutes of Health Research (MOP 97947 & MOP 133652), Canadian Diabetes Association (NC-3-17-5232-CG) and CHU de Québec-Université Laval Foundation provided funding for this research. AMC is the recipient of Fonds de recherche du Québec-Santé (FRQ-S) and Diabetes Canada scholarships. MF holds the Canada Research Chair in Bone and Energy Metabolism. FM has a scholarship from FRQ-S and is a co-Chair of the Amgen Research Chair in Nephrology from Université Laval Foundation. CG is a clinical research scholar of the FRQ-S and the recipient of a Diabetes Canada New Investigator Award.
Author information
Authors and Affiliations
Contributions
A.F.T. and C.G. contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.F.T., T.G.L., J.L., A.C.C., M.F. and C.G. The first draft of the paper was written by A.F.T. and all authors commented on previous versions of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
A.M.C. receives consultation honoraria from Pfizer. A.C.C. is the recipient of the Canada Research Chair in Molecular Imaging of Diabetes. A.T. and L.B. receive funding from Johnson Johnson Medical Companies and Medtronic for research studies on bariatric surgery. M.F. receives royalties from BioLegend for the development of the ucOCN ELISA assay. T.G.L. is now a full-time employee of Boehringer Ingelheim Canada. F.M. received speaker honoraria from Amgen and Sanofi, and participated in advisory committee for Otsuka. C.G. received research funding from Shire and speaker honoraria from Amgen, Eli Lilly and Janssen.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institut Universitaire de cardiologie et de pneumologie de Québec (IUCPQ) ethical review board.
Informed consent
Informed consent was obtained for all participants before entering the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turcotte, AF., Grenier-Larouche, T., Lacombe, J. et al. Association between changes in bioactive osteocalcin and glucose homeostasis after biliopancreatic diversion. Endocrine 69, 526–535 (2020). https://doi.org/10.1007/s12020-020-02340-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02340-2