Abstract
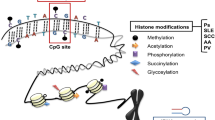
Epigenetics is the study of heritable, reversible gene expression patterns that do not originate from alterations in the DNA sequence. Epigenetic modifications influence gene expression patterns and include DNA methylation, histone modifications, and gene regulation via non-coding RNAs. While the study of epigenetics has been most broadly applied to neoplastic diseases, the role of the epigenome in a wide range of disease processes including autoimmune, allergic, and inflammatory processes is increasingly being recognized. Recent advances in the study of the epigenome have led to novel insights into the pathogenesis and potential therapeutic targets of various pathologic entities including inflammatory diseases. In this review, we examine the nature of epigenetic modifications in several well-studied autoimmune, allergic, and/or inflammatory disorders of the skin including systemic lupus erythematosus, vitiligo, systemic sclerosis, alopecia areata, pemphigus, psoriasis, atopic dermatitis, keloidal scarring, and hidradenitis suppurativa with the aim to determine how such epigenetic changes may be targeted for therapeutic benefit.

Similar content being viewed by others
References
Hirst M, Marra MA (2009) Epigenetics and human disease. Int J Biochem Cell Biol 41(1):136–146. https://doi.org/10.1016/j.biocel.2008.09.011
Holliday R (2006) Epigenetics: a historical overview. Epigenetics 1(2):76–80. https://doi.org/10.4161/epi.1.2.2762
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447(7143):433–440. https://doi.org/10.1038/nature05919
Dawson MA, Kouzarides T (2012) Cancer epigenetics: from mechanism to therapy. Cell 150(1):12–27. https://doi.org/10.1016/j.cell.2012.06.013
Arozarena I, Wellbrock C (2019) Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat Rev Cancer 19(7):377–391. https://doi.org/10.1038/s41568-019-0154-4
Kim E, Zucconi BE, Wu M et al (2019) MITF expression predicts therapeutic vulnerability to p300 inhibition in human melanoma. Cancer Res 79(10):2649–2661. https://doi.org/10.1158/0008-5472.can-18-2331
Yu Y, Schleich K, Yue B et al (2018) Targeting the senescence-overriding cooperative activity of structurally unrelated H3K9 demethylases in melanoma. Cancer Cell 33(2):322–336.e8. https://doi.org/10.1016/j.ccell.2018.01.002
Ceol CJ, Houvras Y, Jane-Valbuena J et al (2011) The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature 471(7339):513–517. https://doi.org/10.1038/nature09806
Shanmugam MK, Sethi G (2013) Role of epigenetics in inflammation-associated diseases. Subcell Biochem 61:627–657. https://doi.org/10.1007/978-94-007-4525-4_27
Hewagama A, Richardson B (2009) The genetics and epigenetics of autoimmune diseases. J Autoimmun 33(1):3–11. https://doi.org/10.1016/j.jaut.2009.03.007
Robertson KD (2005) DNA methylation and human disease. Nat Rev Genet 6(8):597–610. https://doi.org/10.1038/nrg1655
Chahrour M, Jung SY, Shaw C et al (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320(5880):1224–1229. https://doi.org/10.1126/science.1153252
Portela A, Esteller M (2010) Epigenetic modifications and human disease. Nat Biotechnol 28(10):1057–1068. https://doi.org/10.1038/nbt.1685
Greer EL, Shi Y (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13(5):343–357. https://doi.org/10.1038/nrg3173
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874. https://doi.org/10.1038/nrg3074
Shi X, Sun M, Liu H et al (2013) Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett 339(2):159–166. https://doi.org/10.1016/j.canlet.2013.06.013
Yu C-Y, Kuo H-C (2019) The emerging roles and functions of circular RNAs and their generation. J Biomed Sci 26(1):29. https://doi.org/10.1186/s12929-019-0523-z
Zhou Y, Kong Y, Fan W et al (2020) Principles of RNA methylation and their implications for biology and medicine. Biomed Pharmacother 131:110731. https://doi.org/10.1016/j.biopha.2020.110731
Mansuri MS, Singh M, Dwivedi M et al (2014) MicroRNA profiling reveals differentially expressed microRNA signatures from the skin of patients with nonsegmental vitiligo. Br J Dermatol 171(5):1263–1267. https://doi.org/10.1111/bjd.13109
Wang M, Chen H, Qiu J et al (2020) Antagonizing miR-7 suppresses B cell hyperresponsiveness and inhibits lupus development. J Autoimmun 109:102440. https://doi.org/10.1016/j.jaut.2020.102440
Malaab M, Renaud L, Takamura N et al (2022) Antifibrotic factor KLF4 is repressed by the miR-10/TFAP2A/TBX5 axis in dermal fibroblasts: insights from twins discordant for systemic sclerosis. Ann Rheum Dis 81(2):268–277. https://doi.org/10.1136/annrheumdis-2021-221050
Shi YL, Weiland M, Li J et al (2013) MicroRNA expression profiling identifies potential serum biomarkers for non-segmental vitiligo. Pigment Cell Melanoma Res 26(3):418–421. https://doi.org/10.1111/pcmr.12086
Yao Q, Xing Y, Wang Z et al (2020) MiR-16–5p suppresses myofibroblast activation in systemic sclerosis by inhibiting NOTCH signaling. Aging (Albany NY) 13(2):2640–2654. https://doi.org/10.18632/aging.202308
Masalha M, Sidi Y, Avni D (2018) The contribution of feedback loops between miRNAs, cytokines and growth factors to the pathogenesis of psoriasis. Exp Dermatol 27(6):603–610. https://doi.org/10.1111/exd.13520
Kaga H, Komatsuda A, Omokawa A et al (2015) Downregulated expression of miR-155, miR-17, and miR-181b, and upregulated expression of activation-induced cytidine deaminase and interferon-alpha in PBMCs from patients with SLE. Mod Rheumatol 25(6):865–70. https://doi.org/10.3109/14397595.2015.1030102
Aguennouz MH, Guarneri F, Oteri R et al (2021) Serum levels of miRNA-21–5p in vitiligo patients and effects of miRNA-21–5p on SOX5, beta-catenin, CDK2 and MITF protein expression in normal human melanocytes. J Dermatol Sci 101(1):22–29. https://doi.org/10.1016/j.jdermsci.2020.10.014
Suo QF, Sheng J, Qiang FY et al (2018) Association of long non-coding RNA GAS5 and miR-21 levels in CD4(+) T cells with clinical features of systemic lupus erythematosus. Exp Ther Med 15(1):345–350. https://doi.org/10.3892/etm.2017.5429
Meisgen F, Xu N, Wei T et al (2012) MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol 21(4):312–314. https://doi.org/10.1111/j.1600-0625.2012.01462.x
Zhu H, Luo H, Li Y et al (2013) MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol 33(6):1100–1109. https://doi.org/10.1007/s10875-013-9896-z
Liu Y, Wang X, Yang D et al (2014) MicroRNA-21 affects proliferation and apoptosis by regulating expression of PTEN in human keloid fibroblasts. Plast Reconstr Surg 134(4):561e–e573. https://doi.org/10.1097/PRS.0000000000000577
Sonkoly E, Wei T, Janson PC et al (2007) MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2(7):e610. https://doi.org/10.1371/journal.pone.0000610
Hessam S, Sand M, Skrygan M et al (2017) Expression of miRNA-155, miRNA-223, miRNA-31, miRNA-21, miRNA-125b, and miRNA-146a in the inflammatory pathway of hidradenitis suppurativa. Inflammation 40(2):464–472. https://doi.org/10.1007/s10753-016-0492-2
Li C, Bai Y, Liu H et al (2013) Comparative study of microRNA profiling in keloid fibroblast and annotation of differential expressed microRNAs. Acta Biochim Biophys Sin (Shanghai) 45(8):692–699. https://doi.org/10.1093/abbs/gmt057
Ciechomska M, Wojtas B, Swacha M et al (2020) Global miRNA and mRNA expression profiles identify miRNA-26a-2–3p-dependent repression of IFN signature in systemic sclerosis human monocytes. Eur J Immunol 50(7):1057–1066. https://doi.org/10.1002/eji.201948428
Henderson J, Wilkinson S, Przyborski S et al (2021) microRNA27a-3p mediates reduction of the Wnt antagonist sFRP-1 in systemic sclerosis. Epigenetics 16(7):808–817. https://doi.org/10.1080/15592294.2020.1827715
Maurer B, Stanczyk J, Jungel A et al (2010) MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62(6):1733–1743. https://doi.org/10.1002/art.27443
Tafazzoli A, Forstner AJ, Broadley D et al (2018) Genome-wide microRNA analysis implicates miR-30b/d in the etiology of Alopecia areata. J Invest Dermatol 138(3):549–556. https://doi.org/10.1016/j.jid.2017.09.046
Huang RY, Li L, Wang MJ et al (2015) An exploration of the role of microRNAs in psoriasis: a systematic review of the literature. Medicine (Baltimore) 94(45):e2030. https://doi.org/10.1097/MD.0000000000002030
Henderson J, Pryzborski S, Stratton R et al (2021) Wnt antagonist DKK-1 levels in systemic sclerosis are lower in skin but not in blood and are regulated by microRNA33a-3p. Exp Dermatol 30(1):162–168. https://doi.org/10.1111/exd.14136
Wasson CW, Abignano G, Hermes H et al (2020) Long non-coding RNA HOTAIR drives EZH2-dependent myofibroblast activation in systemic sclerosis through miRNA 34a-dependent activation of NOTCH. Ann Rheum Dis 79(4):507–517. https://doi.org/10.1136/annrheumdis-2019-216542
Xu N, Brodin P, Wei T et al (2011) MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol 131(7):1521–1529. https://doi.org/10.1038/jid.2011.55
Tili E, Michaille JJ, Cimino A et al (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179(8):5082–5089. https://doi.org/10.4049/jimmunol.179.8.5082
Sheng Y, Qi S, Hu R et al (2019) Identification of blood microRNA alterations in patients with severe active alopecia areata. J Cell Biochem 120(9):14421–14430. https://doi.org/10.1002/jcb.28700
Chouri E, Wang M, Hillen MR et al (2021) Implication of miR-126 and miR-139–5p in plasmacytoid dendritic cell dysregulation in systemic sclerosis. J Clin Med 10(3):491. https://doi.org/10.3390/jcm10030491
Feng S, Wang L, Liu W et al (2018) MiR-126 correlates with increased disease severity and promotes keratinocytes proliferation and inflammation while suppresses cells’ apoptosis in psoriasis. J Clin Lab Anal 32(9):e22588. https://doi.org/10.1002/jcla.22588
Zhao S, Wang Y, Liang Y et al (2011) MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum 63(5):1376–1386. https://doi.org/10.1002/art.30196
Wang Y, Liang J, Qin H et al (2016) Elevated expression of miR-142-3p is related to the pro-inflammatory function of monocyte-derived dendritic cells in SLE. Arthritis Res Ther 18(1):263. https://doi.org/10.1186/s13075-016-1158-z
Løvendorf MB, Zibert JR, Gyldenløve M et al (2014) MicroRNA-223 and miR-143 are important systemic biomarkers for disease activity in psoriasis. J Dermatol Sci 75(2):133–139. https://doi.org/10.1016/j.jdermsci.2014.05.005
Xia P, Fang X, Zhang ZH et al (2012) Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol Lett 148(2):151–162. https://doi.org/10.1016/j.imlet.2012.09.004
Tang Y, Luo X, Cui H et al (2009) MicroRNA-146a contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheumatism 60(4):1065–1075. https://doi.org/10.1002/art.24436
Yan Q, Chen J, Li W et al (2016) Targeting miR-155 to treat experimental scleroderma. Sci Rep 6:20314. https://doi.org/10.1038/srep20314
Sonkoly E, Janson P, Majuri ML et al (2010) MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol 126(3):581–9.e1-20
Iwamoto N, Vettori S, Maurer B et al (2016) Downregulation of miR-193b in systemic sclerosis regulates the proliferative vasculopathy by urokinase-type plasminogen activator expression. Ann Rheum Dis 75(1):303–310. https://doi.org/10.1136/annrheumdis-2014-205326
Zhao M, Wang LT, Liang GP et al (2014) Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol 150(1):22–30. https://doi.org/10.1016/j.clim.2013.10.009
Kashiyama K, Mitsutake N, Matsuse M et al (2012) miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J Invest Dermatol 132(6):1597–1604. https://doi.org/10.1038/jid.2012.22
Wu ZY, Lu L, Liang J et al (2014) Keloid microRNA expression analysis and the influence of miR-199a-5p on the proliferation of keloid fibroblasts. Genet Mol Res 13(2):2727–2738. https://doi.org/10.4238/2014.April.14.2
Wang Y, Wang K, Liang J et al (2015) Differential expression analysis of miRNA in peripheral blood mononuclear cells of patients with non-segmental vitiligo. J Dermatol 42(2):193–197. https://doi.org/10.1111/1346-8138.12725
Sun XG, Tao JH, Xiang N et al (2016) Negative correlation between miR-326 and Ets-1 in regulatory T cells from new-onset SLE patients. Inflammation 39(2):822–829. https://doi.org/10.1007/s10753-016-0312-8
Liu Q, Cui F, Wang M et al (2018) Increased expression of microRNA-338-3p contributes to production of Dsg3 antibody in pemphigus vulgaris patients. Mol Med Rep 18(1):550–556. https://doi.org/10.3892/mmr.2018.8934
Wang M, Liang L, Li L et al (2017) Increased miR-424-5p expression in peripheral blood mononuclear cells from patients with pemphigus. Mol Med Rep 15(6):3479–3484. https://doi.org/10.3892/mmr.2017.6422
Jiang M, Sun Z, Dang E et al (2017) TGFbeta/SMAD/microRNA-486-3p signaling axis mediates keratin 17 expression and keratinocyte hyperproliferation in psoriasis. J Invest Dermatol 137(10):2177–2186. https://doi.org/10.1016/j.jid.2017.06.005
Rossato M, Affandi AJ, Thordardottir S et al (2017) Association of microRNA-618 expression with altered frequency and activation of plasmacytoid dendritic cells in patients with systemic sclerosis. Arthritis Rheumatol 69(9):1891–1902. https://doi.org/10.1002/art.40163
Ueta M, Nishigaki H, Komai S et al (2021) Difference in the plasma level of miR-628–3p in atopic dermatitis patients with/without atopic keratoconjunctivitis. Immun Inflamm Dis 9(4):1815–1819. https://doi.org/10.1002/iid3.536
Seifeldin NS, El Sayed SB, Asaad MK (2016) Increased microRNA-1266 levels as a biomarker for disease activity in psoriasis vulgaris. Int J Dermatol 55(11):1242–1247. https://doi.org/10.1111/ijd.13102
Liu P, Hu Y, Xia L et al (2020) miR-4417 suppresses keloid fibrosis growth by inhibiting CyclinD1. J Biosci 45:47. https://doi.org/10.1007/s12038-020-0018-9
Feinberg AP, Fallin MD (2015) Epigenetics at the crossroads of genes and the environment. JAMA 314(11):1129–1130. https://doi.org/10.1001/jama.2015.10414
Wu H, Zhao M, Chang C et al (2015) The real culprit in systemic lupus erythematosus: abnormal epigenetic regulation. Int J Mol Sci 16(5):11013–11033. https://doi.org/10.3390/ijms160511013
Javierre BM, Fernandez AF, Richter J et al (2010) Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res 20(2):170–179. https://doi.org/10.1101/gr.100289.109
Marion MC, Ramos PS, Bachali P et al (2021) Nucleic acid-sensing and interferon-inducible pathways show differential methylation in MZ twins discordant for lupus and overexpression in independent lupus samples: implications for pathogenic mechanism and drug targeting. Genes 12(12):1898. https://doi.org/10.3390/genes12121898
Teruel M, Sawalha AH (2017) Epigenetic variability in systemic lupus erythematosus: what we learned from genome-wide DNA methylation studies. Curr Rheumatol Rep 19(6):32. https://doi.org/10.1007/s11926-017-0657-5
Fang H, Disteche CM, Berletch JB (2019) X inactivation and escape: epigenetic and structural features. Front Cell Dev Biol 7:219. https://doi.org/10.3389/fcell.2019.00219
Pyfrom S, Paneru B, Knox James J et al (2021) The dynamic epigenetic regulation of the inactive X chromosome in healthy human B cells is dysregulated in lupus patients. Proc Natl Acad Sci 118(24):e2024624118. https://doi.org/10.1073/pnas.2024624118
Leung YT, Maurer K, Song L et al (2020) Prolactin activates IRF1 and leads to altered balance of histone acetylation: implications for systemic lupus erythematosus. Mod Rheumatol 30(3):532–543. https://doi.org/10.1080/14397595.2019.1620999
Richardson B, Scheinbart L, Strahler J et al (1990) Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 33(11):1665–1673. https://doi.org/10.1002/art.1780331109
Jeffries MA, Dozmorov M, Tang Y et al (2011) Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics 6(5):593–601. https://doi.org/10.4161/epi.6.5.15374
Lei W, Luo Y, Lei W et al (2009) Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand J Rheumatol 38(5):369–374. https://doi.org/10.1080/03009740902758875
Absher DM, Li X, Waite LL et al (2013) Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet 9(8):e1003678. https://doi.org/10.1371/journal.pgen.1003678
Coit P, Yalavarthi S, Ognenovski M et al (2015) Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J Autoimmun 58:59–66. https://doi.org/10.1016/j.jaut.2015.01.004
Crow MK (2014) Type I interferon in the pathogenesis of lupus. J Immunol 192(12):5459–5468. https://doi.org/10.4049/jimmunol.1002795
Zhang B, Zhou T, Wu H et al (2021) Difference of IFI44L methylation and serum IFN-a1 level among patients with discoid and systemic lupus erythematosus and healthy individuals. J Transl Autoimmun 4:100092. https://doi.org/10.1016/j.jtauto.2021.100092
Gautam P, Sharma A, Bhatnagar A (2021) Global histone modification analysis reveals hypoacetylated H3 and H4 histones in B cells from systemic lupus erythematosus patients. Immunol Lett 240:41–45. https://doi.org/10.1016/j.imlet.2021.09.007
Yang M, Long D, Hu L et al (2021) AIM2 deficiency in B cells ameliorates systemic lupus erythematosus by regulating Blimp-1–Bcl-6 axis-mediated B-cell differentiation. Signal Transduct Target Ther 6(1):341. https://doi.org/10.1038/s41392-021-00725-x
Breitbach ME, Ramaker RC, Roberts K et al (2020) Population-specific patterns of epigenetic defects in the B cell lineage in patients with systemic lupus erythematosus. Arthritis Rheumatol 72(2):282–291. https://doi.org/10.1002/art.41083
Vordenbäumen S, Rosenbaum A, Gebhard C et al (2021) Associations of site-specific CD4(+)-T-cell hypomethylation within CD40-ligand promotor and enhancer regions with disease activity of women with systemic lupus erythematosus. Lupus 30(1):45–51. https://doi.org/10.1177/0961203320965690
Lu Q, Wu A, Tesmer L et al (2007) Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol 179(9):6352–6358. https://doi.org/10.4049/jimmunol.179.9.6352
Lu Q, Wu A, Ray D et al (2003) DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol 170(10):5124–5132. https://doi.org/10.4049/jimmunol.170.10.5124
Hanaei S, Sanati G, Zoghi S et al (2020) The status of FOXP3 gene methylation in pediatric systemic lupus erythematosus. Allergol Immunopathol (Madr) 48(4):332–338. https://doi.org/10.1016/j.aller.2020.03.014
Zhang Q, Liang Y, Yuan H et al (2019) Integrated analysis of lncRNA, miRNA and mRNA expression profiling in patients with systemic lupus erythematosus. Arch Med Sci 15(4):872–879. https://doi.org/10.5114/aoms.2018.79145
Sheedy FJ, Palsson-McDermott E, Hennessy EJ et al (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11(2):141–147. https://doi.org/10.1038/ni.1828
Cheng Y, Ji R, Yue J et al (2007) MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol 170(6):1831–1840. https://doi.org/10.2353/ajpath.2007.061170
Liu Y, Yang D, Xiao Z et al (2012) miRNA expression profiles in keloid tissue and corresponding normal skin tissue. Aesthetic Plast Surg 36(1):193–201. https://doi.org/10.1007/s00266-011-9773-1
Lee J, Jang A, Seo SJ et al (2020) Epigenetic regulation of filaggrin gene expression in human epidermal keratinocytes. Ann Dermatol 32(2):122–129
Si ML, Zhu S, Wu H et al (2007) miR-21-mediated tumor growth. Oncogene 26(19):2799–2803. https://doi.org/10.1038/sj.onc.1210083
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65(14):6029–6033. https://doi.org/10.1158/0008-5472.CAN-05-0137
Gao X, Song Y, Du P et al (2022) Administration of a microRNA-21 inhibitor improves the lupus-like phenotype in MRL/lpr mice by repressing Tfh cell-mediated autoimmune responses. Int Immunopharmacol 106:108578. https://doi.org/10.1016/j.intimp.2022.108578
Hou G, Harley ITW, Lu X et al (2021) SLE non-coding genetic risk variant determines the epigenetic dysfunction of an immune cell specific enhancer that controls disease-critical microRNA expression. Nat Commun 12(1):135. https://doi.org/10.1038/s41467-020-20460-1
Fouda ME, Nour El Din DM, Mahgoub MY et al (2020) Genetic variants of microRNA-146a gene: an indicator of systemic lupus erythematosus susceptibility, lupus nephritis, and disease activity. Mol Biol Rep 47(10):7459–7466. https://doi.org/10.1007/s11033-020-05802-y
Xie S, Zeng Q, Ouyang S et al (2021) Bioinformatics analysis of epigenetic and SNP-related molecular markers in systemic lupus erythematosus. Am J Transl Res 13(6):6312–6329
Tsao BP (2004) Update on human systemic lupus erythematosus genetics. Curr Opin Rheumatol 16(5):513–521. https://doi.org/10.1097/01.bor.0000132648.62680.81
Johanneson B, Lima G, von Salome J et al (2002) A major susceptibility locus for systemic lupus erythemathosus maps to chromosome 1q31. Am J Hum Genet 71(5):1060–1071. https://doi.org/10.1086/344289
Haywood ME, Rose SJ, Horswell S et al (2006) Overlapping BXSB congenic intervals, in combination with microarray gene expression, reveal novel lupus candidate genes. Genes Immun 7(3):250–263. https://doi.org/10.1038/sj.gene.6364294
Wu GC, Pan HF, Leng RX et al (2015) Emerging role of long noncoding RNAs in autoimmune diseases. Autoimmun Rev 14(9):798–805. https://doi.org/10.1016/j.autrev.2015.05.004
Li J, Wu GC, Zhang TP et al (2017) Association of long noncoding RNAs expression levels and their gene polymorphisms with systemic lupus erythematosus. Sci Rep 7(1):15119. https://doi.org/10.1038/s41598-017-15156-4
Wu Y, Zhang F, Ma J et al (2015) Association of large intergenic noncoding RNA expression with disease activity and organ damage in systemic lupus erythematosus. Arthritis Res Ther 17:131. https://doi.org/10.1186/s13075-015-0632-3
Guo G, Chen A, Ye L et al (2020) TCONS_00483150 as a novel diagnostic biomarker of systemic lupus erythematosus. Epigenomics 12(11):973–988. https://doi.org/10.2217/epi-2019-0265
Wang Z, Zhao M, Yin J et al (2020) E4BP4-mediated inhibition of T follicular helper cell differentiation is compromised in autoimmune diseases. J Clin Investig 130(7):3717–3733. https://doi.org/10.1172/JCI129018
Liu L, Hu L, Yang L et al (2021) UHRF1 downregulation promotes T follicular helper cell differentiation by increasing BCL6 expression in SLE. Clin Epigenetics 13(1):31. https://doi.org/10.1186/s13148-021-01007-7
Wu L, Jiang X, Qi C et al (2021) EZH2 inhibition interferes with the activation of type I interferon signaling pathway and ameliorates lupus nephritis in NZB/NZW F1 mice. Front Immunol 12:653989. https://doi.org/10.3389/fimmu.2021.653989
Zhen Y, Smith RD, Finkelman FD et al (2020) Ezh2-mediated epigenetic modification is required for allogeneic T cell-induced lupus disease. Arthritis Res Ther 22(1):133. https://doi.org/10.1186/s13075-020-02225-9
Hu N, Qiu X, Luo Y et al (2008) Abnormal histone modification patterns in lupus CD4+ T cells. J Rheumatol 35(5):804–810
Garcia BA, Busby SA, Shabanowitz J et al (2005) Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone deacetylase inhibition. J Proteome Res 4(6):2032–2042. https://doi.org/10.1021/pr050188r
Mishra N, Reilly CM, Brown DR et al (2003) Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest 111(4):539–552. https://doi.org/10.1172/JCI16153
Spritz RA (2007) The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res 20(4):271–278. https://doi.org/10.1111/j.1600-0749.2007.00384.x
Sreekumar GP, Erf GF, Smyth JR Jr (1996) 5-azacytidine treatment induces autoimmune vitiligo in parental control strains of the Smyth line chicken model for autoimmune vitiligo. Clin Immunol Immunopathol 81(2):136–144. https://doi.org/10.1006/clin.1996.0169
Zailaie MZ (2005) Decreased proinflammatory cytokine production by peripheral blood mononuclear cells from vitiligo patients following aspirin treatment. Saudi Med J 26(5):799–805
Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ et al (2003) Immunopolarization of CD4+ and CD8+ T cells to type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest 83(5):683–695. https://doi.org/10.1097/01.lab.0000069521.42488.1b
Zhao M, Gao F, Wu X et al (2010) Abnormal DNA methylation in peripheral blood mononuclear cells from patients with vitiligo. Br J Dermatol 163(4):736–742. https://doi.org/10.1111/j.1365-2133.2010.09919.x
Pu Y, Chen X, Chen Y et al (2021) Transcriptome and differential methylation integration analysis identified important differential methylation annotation genes and functional epigenetic modules related to vitiligo. Front Immunol 12:587440. https://doi.org/10.3389/fimmu.2021.587440
Yan S, Shi J, Sun D et al (2020) Current insight into the roles of microRNA in vitiligo. Mol Biol Rep. https://doi.org/10.1007/s11033-020-05336-3
Shang Z, Li H (2017) Altered expression of four miRNA (miR-1238-3p, miR-202-3p, miR-630 and miR-766-3p) and their potential targets in peripheral blood from vitiligo patients. J Dermatol 44(10):1138–1144. https://doi.org/10.1111/1346-8138.13886
Vaish U, Kumar AA, Varshney S et al (2019) Micro RNAs upregulated in Vitiligo skin play an important role in its aetiopathogenesis by altering TRP1 expression and keratinocyte-melanocytes cross-talk. Sci Rep 9(1):10079. https://doi.org/10.1038/s41598-019-46529-6
Mueller DW, Rehli M, Bosserhoff AK (2009) miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol 129(7):1740–1751. https://doi.org/10.1038/jid.2008.452
Jiang S, Li C, Olive V et al (2011) Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood 118(20):5487–5497. https://doi.org/10.1182/blood-2011-05-355644
Paraskevi A, Theodoropoulos G, Papaconstantinou I et al (2012) Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis 6(9):900–904. https://doi.org/10.1016/j.crohns.2012.02.006
Shi YL, Weiland M, Lim HW et al (2014) Serum miRNA expression profiles change in autoimmune vitiligo in mice. Exp Dermatol 23(2):140–142. https://doi.org/10.1111/exd.12319
Pauley KM, Satoh M, Chan AL et al (2008) Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther 10(4):R101. https://doi.org/10.1186/ar2493
Hermann H, Runnel T, Aab A et al (2017) miR-146b Probably Assists miRNA-146a in the suppression of keratinocyte proliferation and inflammatory responses in psoriasis. J Invest Dermatol 137(9):1945–1954. https://doi.org/10.1016/j.jid.2017.05.012
Harris JE, Harris TH, Weninger W et al (2012) A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol 132(7):1869–1876. https://doi.org/10.1038/jid.2011.463
Kordaß T, Weber CEM, Oswald M et al (2016) SOX5 is involved in balanced MITF regulation in human melanoma cells. BMC Med Genomics 9(1):10. https://doi.org/10.1186/s12920-016-0170-0
Angiolilli C, Marut W, van der Kroef M et al (2018) New insights into the genetics and epigenetics of systemic sclerosis. Nat Rev Rheumatol 14(11):657–673. https://doi.org/10.1038/s41584-018-0099-0
Yi X, Guo W, Shi Q et al (2019) SIRT3-dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics 9(6):1614–1633. https://doi.org/10.7150/thno.30398
Becatti M, Fiorillo C, Barygina V et al (2014) SIRT1 regulates MAPK pathways in vitiligo skin: insight into the molecular pathways of cell survival. J Cell Mol Med 18(3):514–529. https://doi.org/10.1111/jcmm.12206
Broen JC, Radstake TR, Rossato M (2014) The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat Rev Rheumatol 10(11):671–681. https://doi.org/10.1038/nrrheum.2014.128
Feghali-Bostwick C, Medsger TA Jr, Wright TM (2003) Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum 48(7):1956–1963. https://doi.org/10.1002/art.11173
De Martinis M, Ciccarelli F, Sirufo MM et al (2016) An overview of environmental risk factors in systemic sclerosis. Expert Rev Clin Immunol 12(4):465–478. https://doi.org/10.1586/1744666X.2016.1125782
Chifflot H, Fautrel B, Sordet C et al (2008) Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 37(4):223–235. https://doi.org/10.1016/j.semarthrit.2007.05.003
Jiang H, Xiao R, Lian X et al (2012) Demethylation of TNFSF7 contributes to CD70 overexpression in CD4+ T cells from patients with systemic sclerosis. Clin Immunol 143(1):39–44. https://doi.org/10.1016/j.clim.2012.01.005
Lian X, Xiao R, Hu X et al (2012) DNA demethylation of CD40l in CD4+ T cells from women with systemic sclerosis: a possible explanation for female susceptibility. Arthritis Rheum 64(7):2338–2345. https://doi.org/10.1002/art.34376
Komura K, Fujimoto M, Yanaba K et al (2008) Blockade of CD40/CD40 ligand interactions attenuates skin fibrosis and autoimmunity in the tight-skin mouse. Ann Rheum Dis 67(6):867–872. https://doi.org/10.1136/ard.2007.073387
Wang Y, Shu Y, Xiao Y et al (2014) Hypomethylation and overexpression of ITGAL (CD11a) in CD4(+) T cells in systemic sclerosis. Clin Epigenetics 6(1):25. https://doi.org/10.1186/1868-7083-6-25
Li Z, Li D, Tsun A et al (2015) FOXP3+ regulatory T cells and their functional regulation. Cell Mol Immunol 12(5):558–565. https://doi.org/10.1038/cmi.2015.10
Lal G, Zhang N, van der Touw W et al (2009) Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol 182(1):259–273. https://doi.org/10.4049/jimmunol.182.1.259
Wang YY, Wang Q, Sun XH et al (2014) DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br J Dermatol 171(1):39–47. https://doi.org/10.1111/bjd.12913
Ding W, Pu W, Wang L et al (2018) Genome-wide DNA methylation analysis in systemic sclerosis reveals hypomethylation of IFN-associated genes in CD4(+) and CD8(+) T cells. J Invest Dermatol 138(5):1069–1077. https://doi.org/10.1016/j.jid.2017.12.003
Yin H, Wu H, Zhao M et al (2017) Histone demethylase JMJD3 regulates CD11a expression through changes in histone H3K27 tri-methylation levels in CD4+ T cells of patients with systemic lupus erythematosus. Oncotarget 8(30):48938–48947. https://doi.org/10.18632/oncotarget.16894
Ghosh AK, Bhattacharyya S, Lafyatis R et al (2013) p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-beta: epigenetic feed-forward amplification of fibrosis. J Invest Dermatol 133(5):1302–1310. https://doi.org/10.1038/jid.2012.479
Altorok N, Tsou PS, Coit P et al (2015) Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann Rheum Dis 74(8):1612–1620. https://doi.org/10.1136/annrheumdis-2014-205303
Hattori M, Yokoyama Y, Hattori T et al (2015) Global DNA hypomethylation and hypoxia-induced expression of the ten eleven translocation (TET) family, TET1, in scleroderma fibroblasts. Exp Dermatol 24(11):841–846. https://doi.org/10.1111/exd.12767
Wang Y, Fan PS, Kahaleh B (2006) Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum 54(7):2271–2279. https://doi.org/10.1002/art.21948
Dees C, Pötter S, Zhang Y et al (2020) TGF-β–induced epigenetic deregulation of SOCS3 facilitates STAT3 signaling to promote fibrosis. J Clin Investig 130(5):2347–2363. https://doi.org/10.1172/JCI122462
Baker Frost D, da Silveira W, Hazard ES et al (2021) Differential DNA methylation landscape in skin fibroblasts from African Americans with systemic sclerosis. Genes 12(2):129. https://doi.org/10.3390/genes12020129
Mayes MD, Lacey JV Jr, Beebe-Dimmer J et al (2003) Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 48(8):2246–2255. https://doi.org/10.1002/art.11073
Laing TJ, Gillespie BW, Toth MB, Mayes MD, Gallavan RH Jr, Burns CJ, Johanns JR, Cooper BC, Keroack BJ, Wasko MC, Lacey JV Jr, Schottenfeld D (1997) Racial differences in scleroderma among women in Michigan. Arthritis Rheumatism 40(4):734–742. https://doi.org/10.1002/art.1780400421
Henderson J, Brown M, Horsburgh S et al (2019) Methyl cap binding protein 2: a key epigenetic protein in systemic sclerosis. Rheumatology 58(3):527–535. https://doi.org/10.1093/rheumatology/key327
He Y, Tsou PS, Khanna D et al (2018) Methyl-CpG-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann Rheum Dis 77(8):1208–1218. https://doi.org/10.1136/annrheumdis-2018-213022
Jafarinejad-Farsangi S, Farazmand A, Gharibdoost F et al (2016) Inhibition of microRNA-21 induces apoptosis in dermal fibroblasts of patients with systemic sclerosis. Int J Dermatol 55(11):1259–1267. https://doi.org/10.1111/ijd.13308
Zhu H, Li Y, Qu S et al (2012) MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol 32(3):514–522. https://doi.org/10.1007/s10875-011-9647-y
Peng WJ, Tao JH, Mei B et al (2012) MicroRNA-29: a potential therapeutic target for systemic sclerosis. Expert Opin Ther Targets 16(9):875–9. https://doi.org/10.1517/14728222.2012.708339
Jafarinejad-Farsangi S, Farazmand A, Mahmoudi M et al (2015) MicroRNA-29a induces apoptosis via increasing the Bax:Bcl-2 ratio in dermal fibroblasts of patients with systemic sclerosis. Autoimmunity 48(6):369–78. https://doi.org/10.3109/08916934.2015.1030616
Wang Y, Sun J, Kahaleh B (2021) Epigenetic down-regulation of microRNA-126 in scleroderma endothelial cells is associated with impaired responses to VEGF and defective angiogenesis. J Cell Mol Med 25(14):7078–7088. https://doi.org/10.1111/jcmm.16727
Messemaker TC, Chadli L, Cai G et al (2018) Antisense long non-coding RNAs are deregulated in skin tissue of patients with systemic sclerosis. J Invest Dermatol 138(4):826–835. https://doi.org/10.1016/j.jid.2017.09.053
Wang Z, Jinnin M, Nakamura K et al (2016) Long non-coding RNA TSIX is upregulated in scleroderma dermal fibroblasts and controls collagen mRNA stabilization. Exp Dermatol 25(2):131–136. https://doi.org/10.1111/exd.12900
Wasson CW, Ross RL, Wells R et al (2020) Long non-coding RNA HOTAIR induces GLI2 expression through Notch signalling in systemic sclerosis dermal fibroblasts. Arthritis Res Ther 22(1):286. https://doi.org/10.1186/s13075-020-02376-9
Pachera E, Assassi S, Salazar GA et al (2020) Long noncoding RNA H19X is a key mediator of TGF-β-driven fibrosis. J Clin Investig 130(9):4888–4905. https://doi.org/10.1172/jci135439
Abd-Elmawla MA, Hassan M, Elsabagh YA et al (2020) Deregulation of long noncoding RNAs ANCR, TINCR, HOTTIP and SPRY4-IT1 in plasma of systemic sclerosis patients: SPRY4-IT1 as a novel biomarker of scleroderma and its subtypes. Cytokine 133:155124. https://doi.org/10.1016/j.cyto.2020.155124
Mariotti B, Servaas NH, Rossato M et al (2019) The long non-coding RNA NRIR drives IFN-response in monocytes: implication for systemic sclerosis. Front Immunol 10:100. https://doi.org/10.3389/fimmu.2019.00100
Servaas NH, Mariotti B, van der Kroef M et al (2021) Characterization of long non-coding RNAs in systemic sclerosis monocytes: a potential role for PSMB8-AS1 in altered cytokine secretion. Int J Mol Sci 22(9):4365. https://doi.org/10.3390/ijms22094365
Huber LC, Distler JH, Moritz F et al (2007) Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum 56(8):2755–2764. https://doi.org/10.1002/art.22759
Hemmatazad H, Rodrigues HM, Maurer B et al (2009) Histone deacetylase 7, a potential target for the antifibrotic treatment of systemic sclerosis. Arthritis Rheum 60(5):1519–1529. https://doi.org/10.1002/art.24494
Ghosh AK, Yuan W, Mori Y et al (2000) Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 19(31):3546–3555. https://doi.org/10.1038/sj.onc.1203693
Shin JY, Beckett JD, Bagirzadeh R et al (2019) Epigenetic activation and memory at a TGFB2 enhancer in systemic sclerosis. Sci Transl Med 11(497):eaaw0790. https://doi.org/10.1126/scitranslmed.aaw0790
Kramer M, Dees C, Huang J et al (2013) Inhibition of H3K27 histone trimethylation activates fibroblasts and induces fibrosis. Ann Rheum Dis 72(4):614–620. https://doi.org/10.1136/annrheumdis-2012-201615
Wang Q, Xiao Y, Shi Y et al (2015) Overexpression of JMJD3 may contribute to demethylation of H3K27me3 in CD4+ T cells from patients with systemic sclerosis. Clin Immunol 161(2):396–399. https://doi.org/10.1016/j.clim.2015.03.006
Bergmann C, Brandt A, Merlevede B et al (2018) The histone demethylase Jumonji domain-containing protein 3 (JMJD3) regulates fibroblast activation in systemic sclerosis. Ann Rheum Dis 77(1):150–158. https://doi.org/10.1136/annrheumdis-2017-211501
Chu H, Jiang S, Liu Q et al (2018) Sirtuin1 protects against systemic sclerosis-related pulmonary fibrosis by decreasing proinflammatory and profibrotic processes. Am J Respir Cell Mol Biol 58(1):28–39. https://doi.org/10.1165/rcmb.2016-0192OC
Zerr P, Palumbo-Zerr K, Huang J et al (2016) Sirt1 regulates canonical TGF-beta signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis 75(1):226–233. https://doi.org/10.1136/annrheumdis-2014-205740
Wei J, Ghosh AK, Chu H et al (2015) The histone deacetylase sirtuin 1 is reduced in systemic sclerosis and abrogates fibrotic responses by targeting transforming growth factor beta signaling. Arthritis Rheumatol 67(5):1323–1334. https://doi.org/10.1002/art.39061
Wyman AE, Noor Z, Fishelevich R et al (2017) Sirtuin 7 is decreased in pulmonary fibrosis and regulates the fibrotic phenotype of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 312(6):L945–L958. https://doi.org/10.1152/ajplung.00473.2016
Sosulski ML, Gongora R, Feghali-Bostwick C et al (2017) Sirtuin 3 deregulation promotes pulmonary fibrosis. J Gerontol A Biol Sci Med Sci 72(5):595–602. https://doi.org/10.1093/gerona/glw151
Akamata K, Wei J, Bhattacharyya M et al (2016) SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7(43):69321–69336. https://doi.org/10.18632/oncotarget.12504
Zhu X, Chu H, Jiang S et al (2017) Sirt1 ameliorates systemic sclerosis by targeting the mTOR pathway. J Dermatol Sci 87(2):149–158. https://doi.org/10.1016/j.jdermsci.2017.04.013
Rehan M, Kurundkar D, Kurundkar AR et al (2021) Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging 1(2):205–217. https://doi.org/10.1038/s43587-021-00027-5
Tsou PS, Palisoc PJ, Ali M et al (2021) Genome-wide reduction in chromatin accessibility and unique transcription factor footprints in endothelial cells and fibroblasts in scleroderma skin. Arthritis Rheumatol 73(8):1501–1513. https://doi.org/10.1002/art.41694
Wasserman D, Guzman-Sanchez DA, Scott K et al (2007) Alopecia areata. Int J Dermatol 46(2):121–131. https://doi.org/10.1111/j.1365-4632.2007.03193.x
Zhao M, Liang G, Wu X et al (2012) Abnormal epigenetic modifications in peripheral blood mononuclear cells from patients with alopecia areata. Br J Dermatol 166(2):226–273. https://doi.org/10.1111/j.1365-2133.2011.10646.x
Kuwano Y, Fujimoto M, Watanabe R et al (2007) Serum chemokine profiles in patients with alopecia areata. Br J Dermatol 157(3):466–473. https://doi.org/10.1111/j.1365-2133.2007.07943.x
Abdelkader HA, Amin I, Rashed LA et al (2022) Histone deacetylase 1 in patients with alopecia areata and acne vulgaris: an epigenetic alteration. Aust J Dermatol 63(2):e138-e141. https://doi.org/10.1111/ajd.13784
Park M, Jang S, Chung JH et al (2021) Inhibition of class I HDACs preserves hair follicle inductivity in postnatal dermal cells. Sci Rep 11(1):24056. https://doi.org/10.1038/s41598-021-03508-0
Wang EHC, DeStefano GM, Patel AV et al (2017) Identification of differentially expressed miRNAs in alopecia areata that target immune-regulatory pathways. Genes Immun 18(2):100–104. https://doi.org/10.1038/gene.2017.4
Didona D, Paolino G, Di Zenzo G et al (2022) Pemphigus vulgaris: present and future therapeutic strategies. Dermatol Pract Concept 12(1):e2022037. https://doi.org/10.5826/dpc.1201a37
Hammers CM, Stanley JR (2020) Recent advances in understanding pemphigus and bullous pemphigoid. J Invest Dermatol 140(4):733–741. https://doi.org/10.1016/j.jid.2019.11.005
Zhao M, Huang W, Zhang Q et al (2012) Aberrant epigenetic modifications in peripheral blood mononuclear cells from patients with pemphigus vulgaris. Br J Dermatol 167(3):523–531. https://doi.org/10.1111/j.1365-2133.2012.11007.x
Spadoni MB, Bumiller-Bini V, Petzl-Erler ML et al (2020) First glimpse of epigenetic effects on Pemphigus foliaceus. J Invest Dermatol 140(2):488-491.e1. https://doi.org/10.1016/j.jid.2019.07.691
Lin N, Liu Q, Wang M et al (2018) Usefulness of miRNA-338-3p in the diagnosis of pemphigus and its correlation with disease severity. PeerJ 6:e5388. https://doi.org/10.7717/peerj.5388
Lobo-Alves SC, Augusto DG, Magalhaes WCS et al (2019) Long noncoding RNA polymorphisms influence susceptibility to endemic pemphigus foliaceus. Br J Dermatol 181(2):324–331. https://doi.org/10.1111/bjd.17640
Salviano-Silva A, Farias TDJ, Bumiller-Bini V et al (2021) Genetic variability of immune-related lncRNAs: polymorphisms in LINC-PINT and LY86-AS1 are associated with pemphigus foliaceus susceptibility. Exp Dermatol 30(6):831–840. https://doi.org/10.1111/exd.14275
Frezzolini A, Cianchini G, Ruffelli M et al (2004) Interleukin-16 expression and release in bullous pemphigoid. Clin Exp Immunol 137(3):595–600. https://doi.org/10.1111/j.1365-2249.2004.02570.x
Liu Z, Dang E, Li B et al (2018) Dysfunction of CD19+CD24hiCD27+ B regulatory cells in patients with bullous pemphigoid. Sci Rep 8(1):703. https://doi.org/10.1038/s41598-018-19226-z
Purzycka-Bohdan D, Szczerkowska-Dobosz A, Zablotna M et al (2016) Assessment of interleukin 16 serum levels and skin expression in psoriasis patients in correlation with clinical severity of the disease. PLoS One 11(10):e0165577. https://doi.org/10.1371/journal.pone.0165577
Tsai Y-C, Tsai T-F (2017) Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Ther Adv Musculoskelet Dis 9(11):277–294. https://doi.org/10.1177/1759720X17735756
Xue H, Gao L, Wu Y et al (2009) The IL-16 gene polymorphisms and the risk of the systemic lupus erythematosus. Clin Chim Acta 403(1–2):223–225. https://doi.org/10.1016/j.cca.2009.03.016
Abu-Zahab Z, Gad NM, Sabry S et al (2017) CD81 and CD19 as marker of activity in systemic lupus erythematosus disease. Egypt J Immunol 24(2):141–149
Lovinsky-Desir S, Miller RL (2012) Epigenetics, asthma, and allergic diseases: a review of the latest advancements. Curr Allergy Asthma Rep 12(3):211–220. https://doi.org/10.1007/s11882-012-0257-4
Frischknecht L, Vecellio M, Selmi C (2019) The role of epigenetics and immunological imbalance in the etiopathogenesis of psoriasis and psoriatic arthritis. Ther Adv Musculoskelet Dis 11:1759720X19886505. https://doi.org/10.1177/1759720X19886505
Zhang P, Zhao M, Liang G et al (2013) Whole-genome DNA methylation in skin lesions from patients with psoriasis vulgaris. J Autoimmun 41:17–24. https://doi.org/10.1016/j.jaut.2013.01.001
Zhang P, Su Y, Chen H et al (2010) Abnormal DNA methylation in skin lesions and PBMCs of patients with psoriasis vulgaris. J Dermatol Sci 60(1):40–42. https://doi.org/10.1016/j.jdermsci.2010.07.011
Roberson ED, Liu Y, Ryan C et al (2012) A subset of methylated CpG sites differentiate psoriatic from normal skin. J Invest Dermatol 132(3 Pt 1):583–592. https://doi.org/10.1038/jid.2011.348
Yooyongsatit S, Ruchusatsawat K, Noppakun N et al (2015) Patterns and functional roles of LINE-1 and Alu methylation in the keratinocyte from patients with psoriasis vulgaris. J Hum Genet 60(7):349–355. https://doi.org/10.1038/jhg.2015.33
Zhou F, Wang W, Shen C et al (2016) Epigenome-wide association analysis identified nine skin DNA methylation loci for psoriasis. J Invest Dermatol 136(4):779–787. https://doi.org/10.1016/j.jid.2015.12.029
Verma D, Ekman AK, Bivik Eding C et al (2018) Genome-wide DNA methylation profiling identifies differential methylation in uninvolved psoriatic epidermis. J Invest Dermatol 138(5):1088–1093. https://doi.org/10.1016/j.jid.2017.11.036
Gao M, Si X (2018) Rapamycin ameliorates psoriasis by regulating the expression and methylation levels of tropomyosin via ERK1/2 and mTOR pathways in vitro and in vivo. Exp Dermatol 27(10):1112–1119. https://doi.org/10.1111/exd.13745
Ormerod AD, Shah SAA, Copeland P et al (2005) Treatment of psoriasis with topical sirolimus: preclinical development and a randomized, double-blind trial. Br J Dermatol 152(4):758–764. https://doi.org/10.1111/j.1365-2133.2005.06438.x
Chandra A, Senapati S, Roy S et al (2018) Epigenome-wide DNA methylation regulates cardinal pathological features of psoriasis. Clin Epigenetics 10(1):108. https://doi.org/10.1186/s13148-018-0541-9
Han J, Park SG, Bae JB et al (2012) The characteristics of genome-wide DNA methylation in naive CD4+ T cells of patients with psoriasis or atopic dermatitis. Biochem Biophys Res Commun 422(1):157–163. https://doi.org/10.1016/j.bbrc.2012.04.128
Park GT, Han J, Park SG et al (2014) DNA methylation analysis of CD4+ T cells in patients with psoriasis. Arch Dermatol Res 306(3):259–268. https://doi.org/10.1007/s00403-013-1432-8
Gervin K, Vigeland MD, Mattingsdal M et al (2012) DNA methylation and gene expression changes in monozygotic twins discordant for psoriasis: identification of epigenetically dysregulated genes. PLoS Genet 8(1):e1002454. https://doi.org/10.1371/journal.pgen.1002454
Hawkes JE, Nguyen GH, Fujita M et al (2016) microRNAs in psoriasis. J Invest Dermatol 136(2):365–371. https://doi.org/10.1038/JID.2015.409
Liu Q, Wu DH, Han L et al (2017) Roles of microRNAs in psoriasis: immunological functions and potential biomarkers. Exp Dermatol 26(4):359–367. https://doi.org/10.1111/exd.13249
Wang Z-Y, Yan B-X, Zhou Y et al (2021) miRNA profiling of extracellular vesicles reveals biomarkers for psoriasis. J Invest Dermatol 141(1):185–189.e4. https://doi.org/10.1016/j.jid.2020.04.021
Timis TL, Orasan RI (2018) Understanding psoriasis: role of miRNAs. Biomed Rep 9(5):367–374. https://doi.org/10.3892/br.2018.1146
Blauvelt A, Chiricozzi A (2018) The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 55(3):379–390. https://doi.org/10.1007/s12016-018-8702-3
El-Komy M, Amin I, El-Hawary MS et al (2020) Upregulation of the miRNA-155, miRNA-210, and miRNA-20b in psoriasis patients and their relation to IL-17. Int J Immunopathol Pharmacol 34:2058738420933742. https://doi.org/10.1177/2058738420933742
Wu R, Zeng J, Yuan J et al (2018) MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest 128(6):2551–2568. https://doi.org/10.1172/JCI97426
Gupta R, Ahn R, Lai K et al (2016) Landscape of long noncoding RNAs in psoriatic and healthy skin. J Invest Dermatol 136(3):603–609. https://doi.org/10.1016/j.jid.2015.12.009
Ahn R, Gupta R, Lai K et al (2016) Network analysis of psoriasis reveals biological pathways and roles for coding and long non-coding RNAs. BMC Genomics 17(1):841. https://doi.org/10.1186/s12864-016-3188-y
Szegedi K, Sonkoly E, Nagy N et al (2010) The anti-apoptotic protein G1P3 is overexpressed in psoriasis and regulated by the non-coding RNA. PRINS Exp Dermatol 19(3):269–278. https://doi.org/10.1111/j.1600-0625.2010.01066.x
Qiao M, Li R, Zhao X et al (2018) Up-regulated lncRNA-MSX2P1 promotes the growth of IL-22-stimulated keratinocytes by inhibiting miR-6731-5p and activating S100A7. Exp Cell Res 363(2):243–254. https://doi.org/10.1016/j.yexcr.2018.01.014
Li H, Yao Q, Mariscal AG et al (2018) Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat Commun 9(1):1420. https://doi.org/10.1038/s41467-018-03704-z
Zhang P, Su Y, Zhao M et al (2011) Abnormal histone modifications in PBMCs from patients with psoriasis vulgaris. Eur J Dermatol 21(4):552–557. https://doi.org/10.1684/ejd.2011.1383
Blander G, Bhimavarapu A, Mammone T et al (2009) SIRT1 promotes differentiation of normal human keratinocytes. J Invest Dermatol 129(1):41–49. https://doi.org/10.1038/jid.2008.179
DeGregori J, Leone G, Miron A et al (1997) Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci U S A 94(14):7245–7250. https://doi.org/10.1073/pnas.94.14.7245
Tovar-Castillo LE, Cancino-Diaz JC, Garcia-Vazquez F et al (2007) Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol 46(3):239–246. https://doi.org/10.1111/j.1365-4632.2006.02962.x
Larsen FS, Holm NV, Henningsen K (1986) Atopic dermatitis. A genetic-epidemiologic study in a population-based twin sample. J Am Acad Dermatol 15(3):487–94
Rodriguez E, Baurecht H, Wahn AF et al (2014) An integrated epigenetic and transcriptomic analysis reveals distinct tissue-specific patterns of DNA methylation associated with atopic dermatitis. J Invest Dermatol 134(7):1873–1883. https://doi.org/10.1038/jid.2014.87
Liang Y, Wang P, Zhao M et al (2012) Demethylation of the FCER1G promoter leads to FcepsilonRI overexpression on monocytes of patients with atopic dermatitis. Allergy 67(3):424–430. https://doi.org/10.1111/j.1398-9995.2011.02760.x
Liang Y, Chang C, Lu Q (2016) The genetics and epigenetics of atopic dermatitis-filaggrin and other polymorphisms. Clin Rev Allergy Immunol 51(3):315–328. https://doi.org/10.1007/s12016-015-8508-5
Ziyab AH, Karmaus W, Holloway JW et al (2013) DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J Eur Acad Dermatol Venereol 27(3):e420–e423. https://doi.org/10.1111/jdv.12000
Nakamura T, Sekigawa I, Ogasawara H et al (2006) Expression of DNMT-1 in patients with atopic dermatitis. Arch Dermatol Res 298(5):253–256. https://doi.org/10.1007/s00403-006-0682-0
Luo Y, Zhou B, Zhao M et al (2014) Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin Exp Dermatol 39(1):48–53. https://doi.org/10.1111/ced.12206
Wang X, Bao K, Wu P et al (2018) Integrative analysis of lncRNAs, miRNAs, and mRNA-associated ceRNA network in an atopic dermatitis recurrence model. Int J Mol Sci 19(10):3263. https://doi.org/10.3390/ijms19103263
Alaskhar Alhamwe B, Khalaila R, Wolf J et al (2018) Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin Immunol 14:39. https://doi.org/10.1186/s13223-018-0259-4
Nguyen CM, Liao W (2015) Genomic imprinting in psoriasis and atopic dermatitis: a review. J Dermatol Sci 80(2):89–93. https://doi.org/10.1016/j.jdermsci.2015.08.004
Millington GW (2006) Genomic imprinting and dermatological disease. Clin Exp Dermatol 31(5):681–688. https://doi.org/10.1111/j.1365-2230.2006.02233.x
Tsai CH, Ogawa R (2019) Keloid research: current status and future directions. Scars Burn Heal 5:2059513119868659. https://doi.org/10.1177/2059513119868659
Yu X, Li Z, Chan MT et al (2015) microRNA deregulation in keloids: an opportunity for clinical intervention? Cell Prolif 48(6):626–630. https://doi.org/10.1111/cpr.12225
Alghamdi MA, Al-Eitan LN, Stevenson A et al (2020) Secreted factors from keloid keratinocytes modulate collagen deposition by fibroblasts from normal and fibrotic tissue: a pilot study. Biomedicines 8(7):200. https://doi.org/10.3390/biomedicines8070200
Wu ZY, Lu L, Guo XR et al (2013) Identification of differently expressed microRNAs in keloid and pilot study on biological function of miR-199a-5p. Zhonghua Zheng Xing Wai Ke Za Zhi 29(4):279–284
Yan L, Cao R, Liu Y et al (2016) MiR-21-5p links epithelial-mesenchymal transition phenotype with stem-like cell signatures via AKT signaling in keloid keratinocytes. Sci Rep 6(1):28281. https://doi.org/10.1038/srep28281
Wu J, Fang L, Cen Y et al (2019) MiR-21 regulates keloid formation by downregulating Smad7 via the TGF-β/Smad signaling pathway. J Burn Care Res 40(6):809–817. https://doi.org/10.1093/jbcr/irz089
Liang X, Ma L, Long X et al (2015) LncRNA expression profiles and validation in keloid and normal skin tissue. Int J Oncol 47(5):1829–1838. https://doi.org/10.3892/ijo.2015.3177
Zhu HY, Bai WD, Li C et al (2016) Knockdown of lncRNA-ATB suppresses autocrine secretion of TGF-beta2 by targeting ZNF217 via miR-200c in keloid fibroblasts. Sci Rep 6:24728. https://doi.org/10.1038/srep24728
Sun XJ, Wang Q, Guo B et al (2017) Identification of skin-related lncRNAs as potential biomarkers that involved in Wnt pathways in keloids. Oncotarget 8(21):34236–34244. https://doi.org/10.18632/oncotarget.15880
Fitzgerald O’Connor EJ, Badshah II, Addae LY et al (2012) Histone deacetylase 2 is upregulated in normal and keloid scars. J Invest Dermatol 132(4):1293–1296. https://doi.org/10.1038/jid.2011.432
Diao JS, Xia WS, Yi CG et al (2011) Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch Dermatol Res 303(8):573–580. https://doi.org/10.1007/s00403-011-1140-1
Jian X, Qu L, Wang Y et al (2019) Trichostatin A-induced miR30a5p regulates apoptosis and proliferation of keloid fibroblasts via targeting BCL2. Mol Med Rep 19(6):5251–5262. https://doi.org/10.3892/mmr.2019.10185
Bhattacharyya S, Ghosh AK, Pannu J et al (2005) Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor beta. Arthritis Rheum 52(4):1248–1258. https://doi.org/10.1002/art.20996
Russell SB, Russell JD, Trupin KM et al (2010) Epigenetically altered wound healing in keloid fibroblasts. J Invest Dermatol 130(10):2489–2496. https://doi.org/10.1038/jid.2010.162
Jones LR, Young W, Divine G et al (2015) Genome-wide scan for methylation profiles in keloids. Dis Markers 2015:943176. https://doi.org/10.1155/2015/943176
Hessam S, Sand M, Lang K et al (2017) Altered global 5-hydroxymethylation status in hidradenitis suppurativa: support for an epigenetic background. Dermatology 233(2–3):129–135. https://doi.org/10.1159/000478043
Hessam S, Sand M, Skrygan M et al (2016) Inflammation induced changes in the expression levels of components of the microRNA maturation machinery Drosha, Dicer, Drosha co-factor DGRC8 and Exportin-5 in inflammatory lesions of hidradenitis suppurativa patients. J Dermatol Sci 82(3):166–174. https://doi.org/10.1016/j.jdermsci.2016.02.009
Mihara K, Cao X-R, Yen A et al (1989) Cell-cycle dependent regulation of phosphorylation of the human retinoblastoma gene product. Science 246:1300–1303
Hessam S, Sand M, Skrygan M et al (2017) The microRNA effector RNA-induced silencing complex in hidradenitis suppurativa: a significant dysregulation within active inflammatory lesions. Arch Dermatol Res 309(7):557–565. https://doi.org/10.1007/s00403-017-1752-1
Funding
M.W. is supported by a Karin Grunebaum Cancer Foundation Research Award. R.A. is supported by a Melanoma Research Alliance Established Investigator Award (https://doi.org/10.48050/pc.gr.86344) and a Department of Defense Translational Research Award in Melanoma (W81XWH-21–1-0980); M.C. is supported by an MRA Dermatology Fellows Award (https://doi.org/10.48050/pc.gr.147045) and a Department of Defense Translational Research Award in Melanoma (W81XWH-21–1-0980).
Author information
Authors and Affiliations
Contributions
RA and MW contributed to the concepts of the review; FG, AH, NAG, NG, and MC performed the literature search and drafted the review; FG, AH, NG, NAG, MW, MC, and RA contributed to the editing phase of the review.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gibson, F., Hanly, A., Grbic, N. et al. Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases. Clinic Rev Allerg Immunol 63, 447–471 (2022). https://doi.org/10.1007/s12016-022-08956-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-022-08956-8