Abstract
A very important cause of the frustration with drug therapy for central nervous system (CNS) diseases is the failure of drug delivery. The blood–brain barrier (BBB) prevents most therapeutic molecules from entering the brain while maintaining CNS homeostasis. Scientists are keen to develop new brain drug delivery systems to solve this dilemma. Extracellular vesicles (EVs), as a class of naturally derived nanoscale vesicles, have been extensively studied in drug delivery due to their superior properties. This review will briefly present current brain drug delivery strategies, including invasive and non-invasive techniques that target the brain, and the application of nanocarriers developed for brain drug delivery in recent years, especially EVs. The cellular origin of EVs affects the surface protein, size, yield, luminal composition, and other properties of EVs, which are also crucial in determining whether EVs are useful as drug carriers. Stem cell-derived EVs, which inherit the properties of parental cells and avoid the drawbacks of cell therapy, have always been favored by researchers. Thus, in this review, we will focus on the application of stem cell-derived EVs for drug delivery in the CNS. Various nucleic acids, proteins, and small-molecule drugs are loaded into EVs with or without modification and undergo targeted delivery to the brain to achieve their therapeutic effects. In addition, the challenges facing the clinical application of EVs as drug carriers will also be discussed. The directions of future efforts may be to improve drug loading efficiency and precise targeting.
Graphical Abstract




Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADMSCs:
-
Adipose-derived mesenchymal stem cells
- ADSCs:
-
Adipose-derived stem cells
- AMT:
-
Adsorptive-mediated transcytosis
- Axin2:
-
Axis inhibition protein 2
- Aβ:
-
β-Amyloid
- BBB:
-
Blood-brain barrier
- BECs:
-
Brain endothelial cells
- BMSCs:
-
Bone marrow mesenchymal stem cells
- CCL-2:
-
C-C chemokine ligand 2
- CCR-2:
-
C-C chemokine receptor type 2
- CED:
-
Convection enhanced delivery
- CMT:
-
Carrier-mediated transport
- CNS:
-
Central nervous system
- CPP:
-
Cell-penetrating peptide
- CXCR4:
-
CXC motif chemokine receptor type 4
- DALYs:
-
Disability-adjusted life-years
- DLS:
-
Dynamic light scattering
- ECs:
-
Endothelial cells
- EPCs:
-
Endothelial progenitor cells
- ESCs:
-
Embryonic stem cells
- EVs:
-
Extracellular vesicles
- 5-FC:
-
5-Fluorocytosine
- FDA:
-
US Food and Drug Administration
- FUS:
-
Focused ultrasound
- GBM:
-
Glioblastoma multiforme
- GCV:
-
Ganciclovir
- GSCs:
-
Glioma stem cells
- HD:
-
Huntington’s disease
- hiPSCs:
-
Human induced pluripotent stem cells
- HMOX1:
-
Heme oxygenase-1
- HSCs:
-
Hematopoietic stem cells
- HSSP:
-
HMOX1-specific short peptide
- HSVTK:
-
Herpes simplex virus thymidine kinase
- HTT:
-
Huntingtin
- iPSCs:
-
Induced pluripotent stem cells
- ISEV:
-
International Society for Extracellular Vesicles
- MCAO:
-
Middle cerebral artery occlusion
- MPS:
-
Mononuclear phagocytic system
- MRgFUS:
-
Magnetic resonant–guided focused ultrasound
- MSCs:
-
Mesenchymal stem cells
- MSC-EVs:
-
MSC-derived EVs
- MT:
-
Mechanical thrombectomy
- NFT:
-
Neurofibrillary tangles
- NLCs:
-
Nanostructured lipid carriers
- NPs:
-
Nanoparticles
- NSCs:
-
Neural stem cells
- NTA:
-
Nanoparticle tracking analysis
- PBCA:
-
Poly(butylcyanoacrylate)
- PD:
-
Parkinson’s disease
- PEDF:
-
Pigment epithelium-derived factor
- PEG:
-
Polyethylene glycol
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PNPs:
-
Polymeric nanoparticles
- PSCI:
-
Post-stroke cognitive impairment
- PTX:
-
Paclitaxel
- RMT:
-
Receptor-mediated transcytosis
- RVG:
-
Rabies virus glycoprotein
- SCs:
-
Stem cells
- SEC:
-
Size exclusion chromatography
- sEVs:
-
Small extracellular vesicles
- siRNA:
-
Small interfering RNA
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- TfR:
-
Transferrin receptor
- TJ:
-
Tight junction
- TMZ:
-
Temozolomide
- tPAs:
-
Tissue plasminogen activators
- yCD::UPRT:
-
Yeast cytosine deaminase (CD)::uracil phosphoribosyl transferase fusion gene
- Zeb2:
-
Zinc finger E-box binding homeobox 2 protein
References
Collaborators, G. B. D. N. (2019). Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology, 18(5), 459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Gouda, N. A., Elkamhawy, A., Cho, J. (2022). Emerging therapeutic strategies for Parkinson’s disease and future prospects: A 2021 update. Biomedicines. 10(2). https://doi.org/10.3390/biomedicines10020371
Zhang, X., Zhang, X., Gao, H., et al. (2022). Phage display derived peptides for Alzheimer’s disease therapy and diagnosis. Theranostics, 12(5), 2041–2062. https://doi.org/10.7150/thno.68636
Karlawish, J., & Grill, J. D. (2021). The approval of Aduhelm risks eroding public trust in Alzheimer research and the FDA. Nature Reviews. Neurology, 17(9), 523–524. https://doi.org/10.1038/s41582-021-00540-6
Ghosh, D., Sehgal, K., Sodnar, B., et al. (2022). Drug repurposing for stroke intervention. Drug Discovery Today. https://doi.org/10.1016/j.drudis.2022.03.003
Dong, X. (2018). Current strategies for brain drug delivery. Theranostics, 8(6), 1481–1493. https://doi.org/10.7150/thno.21254
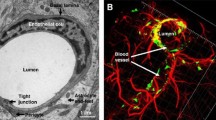
Abbott, N. J., Patabendige, A. A., Dolman, D. E., et al. (2010). Structure and function of the blood-brain barrier. Neurobiology of Diseases, 37(1), 13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Daneman, R., & Prat, A. (2015). The blood-brain barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. https://doi.org/10.1101/cshperspect.a020412
Eichler, A. F., Chung, E., Kodack, D. P., et al. (2011). The biology of brain metastases-translation to new therapies. Nature Reviews. Clinical Oncology, 8(6), 344–356. https://doi.org/10.1038/nrclinonc.2011.58
Han, L., & Jiang, C. (2021). Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharmaceutica Sinica B, 11(8), 2306–2325. https://doi.org/10.1016/j.apsb.2020.11.023
Pardridge, W. M. (2005). The blood-brain barrier: Bottleneck in brain drug development. NeuroRx, 2(1), 3–14. https://doi.org/10.1602/neurorx.2.1.3
Pardridge, W. M. (2007). Drug targeting to the brain. Pharmaceutical Research, 24(9), 1733–1744. https://doi.org/10.1007/s11095-007-9324-2
Wang, Z., Guo, W., Kuang, X., et al. (2017). Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian Journal of Pharmaceutical Sciences, 12(6), 498–508. https://doi.org/10.1016/j.ajps.2017.05.006
Shi, J., Zhang, H., Chen, Z., et al. (2017). A multi-functional nanoplatform for efficacy tumor theranostic applications. Asian Journal of Pharmaceutical Sciences, 12(3), 235–249. https://doi.org/10.1016/j.ajps.2016.12.001
Li, X., Tsibouklis, J., Weng, T., et al. (2017). Nano carriers for drug transport across the blood-brain barrier. Journal of Drug Targeting, 25(1), 17–28. https://doi.org/10.1080/1061186X.2016.1184272
Shahjin, F., Chand, S., & Yelamanchili, S. V. (2020). Extracellular vesicles as drug delivery vehicles to the central nervous system. Journal of Neuroimmune Pharmacology, 15(3), 443–458. https://doi.org/10.1007/s11481-019-09875-w
Karamanidou, T., Tsouknidas, A. (2021) Plant-derived extracellular vesicles as therapeutic nanocarriers. International Journal of Molecular Sciences. 23(1). https://doi.org/10.3390/ijms23010191
Weissman, I. L., Anderson, D. J., & Gage, F. (2001). Stem and progenitor cells: Origins, phenotypes, lineage commitments, and transdifferentiations. Annual Review of Cell and Developmental Biology, 17, 387–403. https://doi.org/10.1146/annurev.cellbio.17.1.387
Okano, H., & Yamanaka, S. (2014). iPS cell technologies: Significance and applications to CNS regeneration and disease. Molecular Brain, 7, 22. https://doi.org/10.1186/1756-6606-7-22
Gao, L., Xu, W., Li, T., et al. (2018). Stem cell therapy: A promising therapeutic method for intracerebral hemorrhage. Cell Transplantation, 27(12), 1809–1824. https://doi.org/10.1177/0963689718773363
De Gioia, R., Biella, F., Citterio, G., et al. (2020). Neural stem cell transplantation for neurodegenerative diseases. International Journal of Molecular Sciences, 21(9), 3103. https://doi.org/10.3390/ijms21093103
Calinescu, A. A., Kauss, M. C., Sultan, Z., et al. (2021). Stem cells for the treatment of glioblastoma: a 20-year perspective. CNS Oncology, 10(2), CNS73. https://doi.org/10.2217/cns-2020-0026
Reis, C., Wilkinson, M., Reis, H., et al. (2017). A look into stem cell therapy: Exploring the options for treatment of ischemic stroke. Stem Cells International, 2017, 3267352. https://doi.org/10.1155/2017/3267352
Koniusz, S., Andrzejewska, A., Muraca, M., et al. (2016). Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Frontiers in Cellular Neuroscience, 10, 109. https://doi.org/10.3389/fncel.2016.00109
Lee, R. H., Pulin, A. A., Seo, M. J., et al. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 5(1), 54–63. https://doi.org/10.1016/j.stem.2009.05.003
Sun, Y., Liu, G., Zhang, K., et al. (2021). Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Research & Therapy, 12(1), 561. https://doi.org/10.1186/s13287-021-02629-7
Yin, L., Liu, X., Shi, Y., et al. (2020)., Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells. 9(3). https://doi.org/10.3390/cells9030707
Ullah, M., Qiao, Y., Concepcion, W., et al. (2019). Stem cell-derived extracellular vesicles: Role in oncogenic processes, bioengineering potential, and technical challenges. Stem Cell Research & Therapy, 10(1), 347. https://doi.org/10.1186/s13287-019-1468-6
Ogawa, K., Kato, N., & Kawakami, S. (2020). Recent strategies for targeted brain drug delivery. Chemical and Pharmaceutical Bulletin (Tokyo), 68(7), 567–582. https://doi.org/10.1248/cpb.c20-00041
Tabet, A., Jensen, M. P., Parkins, C. C., et al. (2019). Designing next-generation local drug delivery vehicles for glioblastoma adjuvant chemotherapy: Lessons from the clinic. Advanced Healthcare Materials, 8(3), e1801391. https://doi.org/10.1002/adhm.201801391
Alghamdi, M., Gumbleton, M., & Newland, B. (2021). Local delivery to malignant brain tumors: Potential biomaterial-based therapeutic/adjuvant strategies. Biomaterials Science, 9(18), 6037–6051. https://doi.org/10.1039/d1bm00896j
Mehta, A. M., Sonabend, A. M., & Bruce, J. N. (2017). Convection-Enhanced Delivery. Neurotherapeutics, 14(2), 358–371. https://doi.org/10.1007/s13311-017-0520-4
Shi, M., & Sanche, L. (2019). Convection-enhanced delivery in malignant gliomas: A review of toxicity and efficacy. Journal of Oncology, 2019, 9342796. https://doi.org/10.1155/2019/9342796
Whish, S., Dziegielewska, K. M., Mollgard, K., et al. (2015). The inner CSF-brain barrier: Developmentally controlled access to the brain via intercellular junctions. Frontiers in Neuroscience, 9, 16. https://doi.org/10.3389/fnins.2015.00016
Rodriguez-Nogales, C., Garbayo, E., Carmona-Abellan, M. M., et al. (2016). Brain aging and Parkinson’s disease: New therapeutic approaches using drug delivery systems. Maturitas, 84, 25–31. https://doi.org/10.1016/j.maturitas.2015.11.009
Gonzalez-Carter, D., Liu, X., Tockary, T. A., et al. (2020). Targeting nanoparticles to the brain by exploiting the blood-brain barrier impermeability to selectively label the brain endothelium. Proceedings of the National Academy of Sciences of the United States of America, 117(32), 19141–19150. https://doi.org/10.1073/pnas.2002016117
Xie, J., Shen, Z., Anraku, Y., et al. (2019). Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials, 224, 119491. https://doi.org/10.1016/j.biomaterials.2019.119491
Chen, Y., & Liu, L. (2012). Modern methods for delivery of drugs across the blood-brain barrier. Advanced Drug Delivery Reviews, 64(7), 640–665. https://doi.org/10.1016/j.addr.2011.11.010
Terstappen, G. C., Meyer, A. H., Bell, R. D., et al. (2021). Strategies for delivering therapeutics across the blood-brain barrier. Nature Reviews. Drug Discovery, 20(5), 362–383. https://doi.org/10.1038/s41573-021-00139-y
Ndemazie, N. B., Inkoom, A., Morfaw, E. F., et al. (2021). Multi-disciplinary approach for drug and gene delivery systems to the brain. An Official Journal of the American Association of Pharmaceutical Scientists, 23(1), 11. https://doi.org/10.1208/s12249-021-02144-1
Anthony, D. P., Hegde, M., Shetty, S. S., et al. (2021). Targeting receptor-ligand chemistry for drug delivery across blood-brain barrier in brain diseases. Life Sciences, 274, 119326. https://doi.org/10.1016/j.lfs.2021.119326
Herve, F., Ghinea, N., & Scherrmann, J. M. (2008). CNS delivery via adsorptive transcytosis. American Association of Pharmaceutical Scientists Journal, 10(3), 455–472. https://doi.org/10.1208/s12248-008-9055-2
Annu, A., Sartaj, Z. Q., et al. (2022). An insight to brain targeting utilizing polymeric nanoparticles: Effective treatment modalities for neurological disorders and brain tumor. Frontiers in Bioengineering and Biotechnology, 10, 788128. https://doi.org/10.3389/fbioe.2022.788128
Kang, Y. C., Son, M., Kang, S., et al. (2018). Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Experimental & Molecular Medicine, 50(8), 1–13. https://doi.org/10.1038/s12276-018-0124-z
Batrakova, E. V., Gendelman, H. E., & Kabanov, A. V. (2011). Cell-mediated drug delivery. Expert Opinion on Drug Delivery, 8(4), 415–433. https://doi.org/10.1517/17425247.2011.559457
Donega, M., Giusto, E., Cossetti, C., et al. (2014). Systemic injection of neural stem/progenitor cells in mice with chronic EAE. Journal of Visualized Experiments, (86). https://doi.org/10.3791/51154
Andreou, T., Rippaus, N., Wronski, K., et al. (2020). Hematopoietic stem cell gene therapy for brain metastases using myeloid cell-specific gene promoters. Journal of the National Cancer Institute, 112(6), 617–627. https://doi.org/10.1093/jnci/djz181
Poon, C., McMahon, D., & Hynynen, K. (2017). Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology, 120, 20–37. https://doi.org/10.1016/j.neuropharm.2016.02.014
Wang, S., Kugelman, T., Buch, A., et al. (2017). Non-invasive, focused ultrasound-facilitated gene delivery for optogenetics. Science and Reports, 7, 39955. https://doi.org/10.1038/srep39955
Meng, Y., Reilly, R. M., Pezo, R. C., et al. (2021). MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Science Translational Medicine, 13(615), eabj4011. https://doi.org/10.1126/scitranslmed.abj4011
Crowe, T. P., Greenlee, M. H. W., Kanthasamy, A. G., et al. (2018). Mechanism of intranasal drug delivery directly to the brain. Life Sciences, 195, 44–52. https://doi.org/10.1016/j.lfs.2017.12.025
Crowe, T. P., & Hsu, W. H. (2022). Evaluation of recent intranasal drug delivery systems to the central nervous system. Pharmaceutics, 14(3), 629. https://doi.org/10.3390/pharmaceutics14030629
Cunha, A., Gaubert, A., Latxague, L., et al. (2021). PLGA-based nanoparticles for neuroprotective drug delivery in neurodegenerative diseases. Pharmaceutics, 13(7), 1042. https://doi.org/10.3390/pharmaceutics13071042
Wilson, B., Samanta, M. K., Santhi, K., et al. (2008). Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Research, 1200, 159–168. https://doi.org/10.1016/j.brainres.2008.01.039
Zhang, W., Mehta, A., Tong, Z., et al. (2021). Development of polymeric nanoparticles for blood-brain barrier transfer-strategies and challenges. Advanced Science (Weinh), 8(10), 2003937. https://doi.org/10.1002/advs.202003937
Gu, J., Al-Bayati, K., & Ho, E. A. (2017). Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Delivery and Translational Research, 7(4), 497–506. https://doi.org/10.1007/s13346-017-0368-5
Ayub, A., & Wettig, S. (2022). An overview of nanotechnologies for drug delivery to the brain. Pharmaceutics, 14(2), 224. https://doi.org/10.3390/pharmaceutics14020224
Xu, C., Nam, J., Hong, H., et al. (2019). Positron emission tomography-guided photodynamic therapy with biodegradable mesoporous silica nanoparticles for personalized cancer immunotherapy. ACS Nano, 13(10), 12148–12161. https://doi.org/10.1021/acsnano.9b06691
Huang, K. W., Hsu, F. F., Qiu, J. T., et al. (2020). Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Science Advances, 6(3), eaax5032. https://doi.org/10.1126/sciadv.aax5032
Gonzalez-Carter, D. A., Ong, Z. Y., McGilvery, C. M., et al. (2019). L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomedicine, 15(1), 1–11. https://doi.org/10.1016/j.nano.2018.08.011
Thomsen, L. B., Linemann, T., Pondman, K. M., et al. (2013). Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chemical Neuroscience, 4(10), 1352–1360. https://doi.org/10.1021/cn400093z
Agrawal, M., Saraf, S., Saraf, S., et al. (2020). Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. Journal of Controlled Release, 321, 372–415. https://doi.org/10.1016/j.jconrel.2020.02.020
Sercombe, L., Veerati, T., Moheimani, F., et al. (2015). Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology, 6, 286. https://doi.org/10.3389/fphar.2015.00286
Juhairiyah, F., & de Lange, E. C. M. (2021). Understanding drug delivery to the brain using liposome-based strategies: Studies that provide mechanistic insights are essential. American Association of Pharmaceutical Scientists Journal, 23(6), 114. https://doi.org/10.1208/s12248-021-00648-z
Vieira, D. B., & Gamarra, L. F. (2016). Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. International Journal of Nanomedicine, 11, 5381–5414. https://doi.org/10.2147/IJN.S117210
Kang, Y. J., Cutler, E. G., & Cho, H. (2018). Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Convergence, 5(1), 35. https://doi.org/10.1186/s40580-018-0168-8
Deatherage, B. L., & Cookson, B. T. (2012). Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infection and Immunity, 80(6), 1948–1957. https://doi.org/10.1128/IAI.06014-11
Robinson, D. G., Ding, Y., & Jiang, L. (2016). Unconventional protein secretion in plants: A critical assessment. Protoplasma, 253(1), 31–43. https://doi.org/10.1007/s00709-015-0887-1
Schorey, J. S., Cheng, Y., Singh, P. P., et al. (2015). Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Reports, 16(1), 24–43. https://doi.org/10.15252/embr.201439363
Johnstone, R. M., Adam, M., Hammond, J. R., et al. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of Biological Chemistry, 262(19), 9412–20.
Mathieu, M., Martin-Jaular, L., Lavieu, G., et al. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology, 21(1), 9–17. https://doi.org/10.1038/s41556-018-0250-9
de Jong, O. G., Kooijmans, S. A. A., Murphy, D. E., et al. (2019). Drug delivery with extracellular vesicles: From imagination to innovation. Accounts of Chemical Research, 52(7), 1761–1770. https://doi.org/10.1021/acs.accounts.9b00109
Caby, M. P., Lankar, D., Vincendeau-Scherrer, C., et al. (2005). Exosomal-like vesicles are present in human blood plasma. International Immunology, 17(7), 879–887. https://doi.org/10.1093/intimm/dxh267
Aalberts, M., van Dissel-Emiliani, F. M. F., van Adrichem, N. P. H., et al. (2012) Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, Annexin A1, and GLIPR2 in humans1. Biology of Reproduction, 86(3). https://doi.org/10.1095/biolreprod.111.095760
Admyre, C., Johansson, S. M., Qazi, K. R., et al. (2007). Exosomes with immune modulatory features are present in human breast milk. The Journal of Immunology, 179(3), 1969–1978. https://doi.org/10.4049/jimmunol.179.3.1969
Pisitkun, T., Shen, R. F., & Knepper, M. A. (2004). Identification and proteomic profiling of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America, 101(36), 13368–13373. https://doi.org/10.1073/pnas.0403453101
Asea, A., Jean-Pierre, C., Kaur, P., et al. (2008). Heat shock protein-containing exosomes in mid-trimester amniotic fluids. Journal of Reproductive Immunology, 79(1), 12–17. https://doi.org/10.1016/j.jri.2008.06.001
Ogawa, Y., Miura, Y., Harazono, A., et al. (2011). Proteomic analysis of two types of exosomes in human whole saliva. Biological &/and Pharmaceutical Bulletin, 34(1), 13–23. https://doi.org/10.1248/bpb.34.13
Vella, L., Sharples, R., Lawson, V., et al. (2007). Packaging of prions into exosomes is associated with a novel pathway of PrP processing. The Journal of Pathology, 211(5), 582–590. https://doi.org/10.1002/path.2145
Bortot, B., Apollonio, M., Rampazzo, E., et al. (2021). Small extracellular vesicles from malignant ascites of patients with advanced ovarian cancer provide insights into the dynamics of the extracellular matrix. Molecular Oncology, 15(12), 3596–3614. https://doi.org/10.1002/1878-0261.13110
Masyuk, A. I., Huang, B. Q., Ward, C. J., et al. (2010). Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. American Journal of Physiology. Gastrointestinal and Liver Physiology, 299(4), G990–G999. https://doi.org/10.1152/ajpgi.00093.2010
van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19(4), 213–228. https://doi.org/10.1038/nrm.2017.125
Sinauridze, E. I., Kireev, D. A., Popenko, N. Y., et al. (2007). Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thrombosis and Haemostasis, 97(03), 425–434.
Xu, R., Rai, A., Chen, M., et al. (2018). Extracellular vesicles in cancer - implications for future improvements in cancer care. Nature Reviews. Clinical Oncology, 15(10), 617–638. https://doi.org/10.1038/s41571-018-0036-9
Robbins, P. D., Dorronsoro, A., & Booker, C. N. (2016). Regulation of chronic inflammatory and immune processes by extracellular vesicles. The Journal of Clinical Investigation, 126(4), 1173–1180. https://doi.org/10.1172/JCI81131
Becker, A., Thakur, B. K., Weiss, J. M., et al. (2016). Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell, 30(6), 836–848. https://doi.org/10.1016/j.ccell.2016.10.009
Samir, E. L. A., Mager, I., Breakefield, X. O., et al. (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery, 12(5), 347–57. https://doi.org/10.1038/nrd3978
Colombo, M., Raposo, G., & Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Kalra, H., Drummen, G. P., & Mathivanan, S. (2016). Focus on extracellular vesicles: Introducing the next small big thing. International Journal of Molecular Sciences, 17(2), 170. https://doi.org/10.3390/ijms17020170
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology, 200(4), 373–383. https://doi.org/10.1083/jcb.201211138
Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science, 367(6478). https://doi.org/10.1126/science.aau6977
Phan, T. K., Ozkocak, D. C., & Poon, I. K. H. (2020). Unleashing the therapeutic potential of apoptotic bodies. Biochemical Society Transactions, 48(5), 2079–2088. https://doi.org/10.1042/BST20200225
Thery, C., Witwer, K. W., Aikawa, E., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. https://doi.org/10.1080/20013078.2018.1535750
Ohnuki, M., & Takahashi, K. (2015). Present and future challenges of induced pluripotent stem cells. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 370(1680), 20140367. https://doi.org/10.1098/rstb.2014.0367
Damdimopoulou, P., Rodin, S., Stenfelt, S., et al. (2016). Human embryonic stem cells. Best Practice & Research. Clinical Obstetrics & Gynaecology, 31, 2–12. https://doi.org/10.1016/j.bpobgyn.2015.08.010
Muller, P., Lemcke, H., & David, R. (2018). Stem cell therapy in heart diseases - cell types, mechanisms and improvement strategies. Cellular Physiology and Biochemistry, 48(6), 2607–2655. https://doi.org/10.1159/000492704
Biressi, S., Filareto, A., & Rando, T. A. (2020). Stem cell therapy for muscular dystrophies. The Journal of Clinical Investigation, 130(11), 5652–5664. https://doi.org/10.1172/JCI142031
Pixley, J. S. (2020). Mesenchymal stem cells to treat type 1 diabetes. Biochimica et Biophysica Acta, Molecular Basis of Disease, 1866(4), 165315. https://doi.org/10.1016/j.bbadis.2018.10.033
Maeda, T., Mandai, M., Sugita, S., et al. (2022). Strategies of pluripotent stem cell-based therapy for retinal degeneration: Update and challenges. Trends in Molecular Medicine, 28(5), 388–404. https://doi.org/10.1016/j.molmed.2022.03.001
Cornelissen, J. J., & Blaise, D. (2016). Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood, 127(1), 62–70. https://doi.org/10.1182/blood-2015-07-604546
Anurogo, D., Yuli Prasetyo Budi, N., Thi Ngo, M. H., et al. (2021). Cell and Gene Therapy for Anemia: Hematopoietic Stem Cells and Gene Editing. International Journal of Molecular Sciences, 22(12). https://doi.org/10.3390/ijms22126275
Ford, E., Pearlman, J., Ruan, T., et al. (2020) Human pluripotent stem cells-based therapies for neurodegenerative diseases: Current status and challenges. Cells, 9(11). https://doi.org/10.3390/cells9112517
Kikuchi, T., Morizane, A., Doi, D., et al. (2017). Idiopathic Parkinson’s disease patient-derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains. Journal of Neuroscience Research, 95(9), 1829–1837. https://doi.org/10.1002/jnr.24014
Hargus, G., Cooper, O., Deleidi, M., et al. (2010). Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proceedings of the National Academy of Sciences of the United States of America, 107(36), 15921–15926. https://doi.org/10.1073/pnas.1010209107
Rhee, Y. H., Ko, J. Y., Chang, M. Y., et al. (2011). Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. Journal of Clinical Investigation, 121(6), 2326–2335. https://doi.org/10.1172/Jci45794
Kikuchi, T., Morizane, A., Doi, D., et al. (2011). Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. Journal of Parkinsons Disease, 1(4), 395–412. https://doi.org/10.3233/Jpd-2011-11070
Marei, H. E., Hasan, A., Rizzi, R., et al. (2018). Potential of stem cell-based therapy for ischemic stroke. Frontiers in Neurology, 9, ARTN 34. https://doi.org/10.3389/fneur.2018.00034
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell, 27(4), 523–531. https://doi.org/10.1016/j.stem.2020.09.014
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology, 8(9), 726–736. https://doi.org/10.1038/nri2395
Huang, L., Ma, W., Ma, Y., et al. (2015). Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? International Journal of Biological Sciences, 11(2), 238–245. https://doi.org/10.7150/ijbs.10725
He, J., Wang, Y., Sun, S., et al. (2012). Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton, Vic.), 17(5), 493–500. https://doi.org/10.1111/j.1440-1797.2012.01589.x
Li, T., Yan, Y., Wang, B., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells and Development, 22(6), 845–854. https://doi.org/10.1089/scd.2012.0395
Allegretta, C., D’Amico, E., Manuti, V., et al. (2022). Mesenchymal stem cell-derived extracellular vesicles and their therapeutic use in central nervous system demyelinating disorders. International Journal of Molecular Sciences, 23(7), 3829.
Caplan, A. I., & Dennis, J. E. (2006). Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry, 98(5), 1076–1084. https://doi.org/10.1002/jcb.20886
Rani, S., Ryan, A. E., Griffin, M. D., et al. (2015). Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Molecular Therapy, 23(5), 812–823. https://doi.org/10.1038/mt.2015.44
You, B., Jin, C., Zhang, J., et al. (2022). MSC-derived extracellular vesicle-delivered L-PGDS inhibit gastric cancer progression by suppressing cancer cell stemness and STAT3 phosphorylation. Stem Cells International, 2022, 9668239. https://doi.org/10.1155/2022/9668239
Harrell, C. R., Jovicic, N., Djonov, V., et al. (2019). Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells, 8(12), 1605.
Lu, V., Tennyson, M., Zhang, J., et al. (2021). Mesenchymal stem cell-derived extracellular vesicles in tendon and ligament repair—A systematic review of in vivo studies. Cells, 10(10), 2553.
Wiklander, O. P. B., Nordin, J. Z., O’Loughlin, A., et al. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of Extracellular Vesicles, 4(1), 26316. https://doi.org/10.3402/jev.v4.26316
Elsharkasy, O. M., Nordin, J. Z., Hagey, D. W., et al. (2020). Extracellular vesicles as drug delivery systems: Why and how? Advanced Drug Delivery Reviews, 159, 332–343. https://doi.org/10.1016/j.addr.2020.04.004
Vader, P., Mol, E. A., Pasterkamp, G., et al. (2016). Extracellular vesicles for drug delivery. Advanced Drug Delivery Reviews, 106, 148–156. https://doi.org/10.1016/j.addr.2016.02.006
Zhuang, X. Y., Xiang, X. Y., Grizzle, W., et al. (2011). Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Molecular Therapy, 19(10), 1769–1779.
Chen, C., Sun, M., Wang, J., et al. (2021). Active cargo loading into extracellular vesicles: Highlights the heterogeneous encapsulation behaviour. Journal of Extracellular Vesicles, 10(13), e12163. https://doi.org/10.1002/jev2.12163
Kim, M. S., Haney, M. J., Zhao, Y., et al. (2016). Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine-Nanotechnology Biology and Medicine, 12(3), 655–664.
Alvarez-Erviti, L., Seow, Y. Q., Yin, H. F., et al. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29(4), 341-U179.
Haney, M. J., Klyachko, N. L., Zhaoa, Y. L., et al. (2015). Exosomes as drug delivery vehicles for Parkinson’s disease therapy. Journal of Controlled Release, 207, 18–30.
Armstrong, J. P. K., Holme, M. N., & Stevens, M. M. (2017). Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano, 11(1), 69–83. https://doi.org/10.1021/acsnano.6b07607
Corso, G., Heusermann, W., Trojer, D., et al. (2019). Systematic characterization of extracellular vesicles sorting domains and quantification at the single molecule - single vesicle level by fluorescence correlation spectroscopy and single particle imaging. Journal of Extracellular Vesicles, 8(1), Artn 1663043. https://doi.org/10.1080/20013078.2019.1663043
Zickler, A. M., & Andaloussi, S. E. L. (2020). Functional extracellular vesicles aplenty. Nature Biomedical Engineering, 4(1), 9–11. https://doi.org/10.1038/s41551-019-0507-z
Tian, Y. H., Li, S. P., Song, J., et al. (2014). A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials, 35(7), 2383–2390.
Mentkowski, K. I. & Lang, J. K. (2019). Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Scientific Reports, 9.
Zhou, M., Wang, H., Zeng, X., et al. (2019). Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet, 394(10204), 1145–1158. https://doi.org/10.1016/s0140-6736(19)30427-1
Benjamin, E. J., Blaha, M. J., Chiuve, S. E., et al. (2017). Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation, 135(10), e146–e603. https://doi.org/10.1161/cir.0000000000000485
Lo, E. H., Dalkara, T., & Moskowitz, M. A. (2003). Mechanisms, challenges and opportunities in stroke. Nature Reviews Neuroscience, 4(5), 399–415. https://doi.org/10.1038/nrn1106
Khoshnam, S. E., Winlow, W., Farzaneh, M., et al. (2017). Pathogenic mechanisms following ischemic stroke. Neurological Sciences, 38(7), 1167–1186. https://doi.org/10.1007/s10072-017-2938-1
Li, Y., Tang, Y., & Yang, G. Y. (2021). Therapeutic application of exosomes in ischaemic stroke. Stroke and Vascular Neurology, 6(3), 483–495. https://doi.org/10.1136/svn-2020-000419
Li, Y., Liu, B., Chen, Y., et al. (2021). Extracellular vesicle application as a novel therapeutic strategy for ischemic stroke. Translational Stroke Research, 13(1), 171–187. https://doi.org/10.1007/s12975-021-00915-3
Xin, H., Li, Y., Cui, Y., et al. (2013). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of Cerebral Blood Flow and Metabolism, 33(11), 1711–1715. https://doi.org/10.1038/jcbfm.2013.152
Bang, O. Y., & Kim, J.-E. (2022). Stem cell-derived extracellular vesicle therapy for acute brain insults and neurodegenerative diseases. BMB Reports, 55(1), 20–29. https://doi.org/10.5483/BMBRep.2022.55.1.162
Zhang, Z. G., & Chopp, M. (2016). Exosomes in stroke pathogenesis and therapy. The Journal of Clinical Investigation, 126(4), 1190–1197. https://doi.org/10.1172/jci81133
Ai, Z., Cheng, C., Zhou, L., et al. (2021). Bone marrow mesenchymal stem cells-derived extracellular vesicles carrying microRNA-221-3p protect against ischemic stroke via ATF3. Brain Research Bulletin, 172, 220–228. https://doi.org/10.1016/j.brainresbull.2021.04.022
Yang, J., Zhang, X., Chen, X., et al. (2017). Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Molecular Therapy - Nucleic Acids, 7, 278–287. https://doi.org/10.1016/j.omtn.2017.04.010
Cheng, C., Chen, X., Wang, Y., et al. (2021). MSCsderived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Molecular Medicine, 27(1), 67. https://doi.org/10.1186/s10020-021-00324-0
Cai, G., Cai, G., Zhou, H., et al. (2021). Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Research & Therapy, 12(1), 2. https://doi.org/10.1186/s13287-020-02030-w
Zhao, Y., Gan, Y., Xu, G., et al. (2020). Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sciences, 260, 118403. https://doi.org/10.1016/j.lfs.2020.118403
Xin, H., Liu, Z., Buller, B., et al. (2021). MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. Journal of Cerebral Blood Flow and Metabolism, 41(5), 1131–1144. https://doi.org/10.1177/0271678X20950489
Pan, Q., Kuang, X., Cai, S., et al. (2020). miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Research & Therapy, 11(1), 260. https://doi.org/10.1186/s13287-020-01761-0
Deng, Y., Chen, D., Gao, F., et al. (2019). Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. Journal of Biological Engineering, 13, 71. https://doi.org/10.1186/s13036-019-0193-0
Xin, H., Katakowski, M., Wang, F., et al. (2017). MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke, 48(3), 747–753. https://doi.org/10.1161/STROKEAHA.116.015204
Xin, H., Li, Y., Liu, Z., et al. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells, 31(12), 2737–2746. https://doi.org/10.1002/stem.1409
Xin, H., Wang, F., Li, Y., et al. (2017). Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from MicroRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplantation, 26(2), 243–257. https://doi.org/10.3727/096368916X693031
Jiang, M., Wang, H., Jin, M., et al. (2018). Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 Microglial/Macrophage polarization. Cellular Physiology and Biochemistry, 47(2), 864–878. https://doi.org/10.1159/000490078
Geng, W., Tang, H., Luo, S., et al. (2019). Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. American Journal of Translational Research, 11(2), 780–792.
Zhang, H., Wu, J., Wu, J., et al. (2019). Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. Journal of Nanobiotechnology, 17(1), 29. https://doi.org/10.1186/s12951-019-0461-7
Wang, J., Chen, S., Zhang, W., et al. (2020). Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neuroscience & Therapeutics, 26(12), 1255–1265. https://doi.org/10.1111/cns.13455
Kalani, A., Chaturvedi, P., Kamat, P. K., et al. (2016). Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. International Journal of Biochemistry & Cell Biology, 79, 360–369. https://doi.org/10.1016/j.biocel.2016.09.002
Tian, T., Zhang, H. X., He, C. P., et al. (2018). Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials, 150, 137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Liu, Y., Fu, N., Su, J., et al. (2019). Rapid enkephalin delivery using exosomes to promote neurons recovery in ischemic stroke by inhibiting neuronal p53/Caspase-3. BioMed Research International, 2019, 4273290. https://doi.org/10.1155/2019/4273290
Li, X., Zhang, Y., Wang, Y., et al. (2020). Exosomes derived from CXCR4-overexpressing BMSC promoted activation of microvascular endothelial cells in cerebral ischemia/reperfusion injury. Neural Plasticity, 2020, 8814239. https://doi.org/10.1155/2020/8814239
Wei, R., Zhang, L., Hu, W., et al. (2022). Zeb2/Axin2-enriched BMSC-derived exosomes promote post-stroke functional recovery by enhancing neurogenesis and neural plasticity. Journal of Molecular Neuroscience, 72(1), 69–81. https://doi.org/10.1007/s12031-021-01887-7
Huang, X., Ding, J., Li, Y., et al. (2018). Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Experimental Cell Research, 371(1), 269–277. https://doi.org/10.1016/j.yexcr.2018.08.021
Yang, H. C., Zhang, M., Wu, R., et al. (2020). C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World Journal of Stem Cells, 12(2), 152–167. https://doi.org/10.4252/wjsc.v12.i2.152
Liu, D. Z., Tian, Y., Ander, B. P., et al. (2010). Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. Journal of Cerebral Blood Flow and Metabolism, 30(1), 92–101. https://doi.org/10.1038/jcbfm.2009.186
Mirzaei, H., Momeni, F., Saadatpour, L., et al. (2018). MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. Journal of Cellular Physiology, 233(2), 856–865. https://doi.org/10.1002/jcp.25787
Chen, H., Wang, L., Zeng, X., et al. (2021). Exosomes, a new star for targeted delivery. Frontiers in Cell and Development Biology, 9, 751079. https://doi.org/10.3389/fcell.2021.751079
Yang, H. H., Chen, Y., Gao, C. Y., et al. (2017). Protective effects of MicroRNA-126 on human cardiac microvascular endothelial cells against Hypoxia/Reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cellular Physiology and Biochemistry, 42(2), 506–518. https://doi.org/10.1159/000477597
Procházka, V., Jurčíková, J., Vítková, K., et al. (2018). The role of miR-126 in critical limb ischemia treatment using adipose-derived stem cell therapeutic factor concentrate and extracellular matrix microparticles. Medical Science Monitor, 24, 511–522. https://doi.org/10.12659/msm.905442
Pan, Q., Zheng, J., Du, D., et al. (2018). MicroRNA-126 priming enhances functions of endothelial progenitor cells under physiological and hypoxic conditions and their therapeutic efficacy in cerebral ischemic damage. Stem Cells International, 2018, 2912347. https://doi.org/10.1155/2018/2912347
Pan, Q., Wang, Y., Lan, Q., et al. (2019). Exosomes derived from mesenchymal stem cells ameliorate Hypoxia/Reoxygenation-injured ECs via transferring MicroRNA-126. Stem Cells International, 2019, 2831756. https://doi.org/10.1155/2019/2831756
Mogilyansky, E., & Rigoutsos, I. (2013). The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death and Differentiation, 20(12), 1603–1614. https://doi.org/10.1038/cdd.2013.125
Geloso, M. C., Corvino, V., Marchese, E., et al. (2017). The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front Aging Neuroscience, 9, 242. https://doi.org/10.3389/fnagi.2017.00242
Becerra, S. P., Sagasti, A., Spinella, P., et al. (1995). Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. The Journal of Biological Chemistry, 270(43), 25992–9. https://doi.org/10.1074/jbc.270.43.25992
Kuo, H. F., Liu, P. L., Chong, I. W., et al. (2016). Pigment epithelium-derived factor mediates autophagy and apoptosis in myocardial Hypoxia/Reoxygenation injury. PLoS ONE, 11(5), e0156059. https://doi.org/10.1371/journal.pone.0156059
Xu, M., Yang, Q., Sun, X., et al. (2020). Recent advancements in the loading and modification of therapeutic exosomes. Frontiers in Bioengineering and Biotechnology, 8, 586130. https://doi.org/10.3389/fbioe.2020.586130
Lee, H. J., Engelhardt, B., Lesley, J., et al. (2000). Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. Journal of Pharmacology and Experimental Therapeutics, 292(3), 1048–1052.
Xie, C. J., Gu, A. P., Cai, J., et al. (2018). Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain and Behavior: A Cognitive Neuroscience Perspective, 8(2), e00921. https://doi.org/10.1002/brb3.921
Liu, Z., Ran, Y., Huang, S., et al. (2017). Curcumin protects against ischemic stroke by titrating microglia/macrophage polarization. Frontiers in Aging Neuroscience, 9, 233. https://doi.org/10.3389/fnagi.2017.00233
Li, W., Suwanwela, N. C., & Patumraj, S. (2017). Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Molecular Medicine Reports, 16(4), 4710–4720. https://doi.org/10.3892/mmr.2017.7205
He, R., Jiang, Y., Shi, Y., et al. (2020). Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Materials Science & Engineering, C: Materials for Biological Applications, 117, 111314. https://doi.org/10.1016/j.msec.2020.111314
(2020). 2020 Alzheimer’s disease facts and figures. Alzheimer's & Dementia. https://doi.org/10.1002/alz.12068
Meldolesi, J. (2019). Alzheimer’s disease: Key developments support promising perspectives for therapy. Pharmacological Research, 146, 104316. https://doi.org/10.1016/j.phrs.2019.104316
Ising, C., & Heneka, M. T. (2018). Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death & Disease, 9(2), 120. https://doi.org/10.1038/s41419-017-0153-x
Bagyinszky, E., Giau, V. V., Shim, K., et al. (2017). Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. Journal of the Neurological Sciences, 376, 242–254. https://doi.org/10.1016/j.jns.2017.03.031
Guo, M., Yin, Z., Chen, F., et al. (2020). Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimer’s Research & Therapy, 12(1), 109. https://doi.org/10.1186/s13195-020-00670-x
Chen, Y. A., Lu, C. H., Ke, C. C., et al. (2021). Mesenchymal stem cell-derived extracellular vesicle-based therapy for Alzheimer’s disease: Progress and opportunity. Membranes (Basel), 11(10), 796. https://doi.org/10.3390/membranes11100796
Jahangard, Y., Monfared, H., Moradi, A., et al. (2020). Therapeutic effects of transplanted exosomes containing miR-29b to a rat model of Alzheimer’s disease. Frontiers in Neuroscience, 14, 564. https://doi.org/10.3389/fnins.2020.00564
Zhai, L., Shen, H., Sheng, Y., et al. (2021). ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer’s disease. Journal of Cellular and Molecular Medicine, 25(15), 7513–7523. https://doi.org/10.1111/jcmm.16787
Izadpanah, M., Dargahi, L., Ai, J., et al. (2020). Extracellular vesicles as a neprilysin delivery system memory improvement in Alzheimer’s disease. Iran Journal of Pharmacy Research, 19(2), 45–60. https://doi.org/10.22037/ijpr.2020.112062.13508
Kalia, L. V., & Lang, A. E. (2015). Parkinson’s disease. Lancet, 386(9996), 896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
Pan, T., Kondo, S., Le, W., et al. (2008). The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain, 131(Pt 8), 1969–1978. https://doi.org/10.1093/brain/awm318
López González, I., Garcia-Esparcia, P., Llorens, F., et al. (2016). Genetic and transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. International Journal of Molecular Sciences, 17(2), 206. https://doi.org/10.3390/ijms17020206
Bai, X., Dong, Q., Zhao, L., et al. (2021). microRNA-106b-containing extracellular vesicles affect autophagy of neurons by regulating CDKN2B in Parkinson’s disease. Neuroscience Letters, 760, 136094. https://doi.org/10.1016/j.neulet.2021.136094
Li, Q., Wang, Z., Xing, H., et al. (2021). Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Molecular Therapy--Nucleic Acids, 23, 1334–1344. https://doi.org/10.1016/j.omtn.2021.01.022
Peng, H., Li, Y., Ji, W., et al. (2022). Intranasal administration of self-oriented nanocarriers based on therapeutic exosomes for synergistic treatment of Parkinson’s disease. ACS Nano. https://doi.org/10.1021/acsnano.1c08473
Joshi, B. S., Youssef, S. A., Bron, R., et al. (2021). DNAJB6b-enriched small extracellular vesicles decrease polyglutamine aggregation in in vitro and in vivo models of Huntington disease. iScience, 24(11), 103282. https://doi.org/10.1016/j.isci.2021.103282
Vigneswaran, K., Neill, S., & Hadjipanayis, C. G. (2015). Beyond the World Health Organization grading of infiltrating gliomas: Advances in the molecular genetics of glioma classification. Annals of Translational Medicine, 3(7), 95. https://doi.org/10.3978/j.issn.2305-5839.2015.03.57
Ostrom, Q. T., Patil, N., Cioffi, G., et al. (2020). CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncology, 22(12 Suppl 2), iv1–iv96. https://doi.org/10.1093/neuonc/noaa200
Hervey-Jumper, S. L., & Berger, M. S. (2016). Maximizing safe resection of low- and high-grade glioma. Journal of Neuro-oncology, 130(2), 269–282. https://doi.org/10.1007/s11060-016-2110-4
Do, A. D., Kurniawati, I., Hsieh, C. L., et al. (2021). Application of mesenchymal stem cells in targeted delivery to the brain: Potential and challenges of the extracellular vesicle-based approach for brain tumor treatment. International Journal of Molecular Sciences, 22(20), 11187. https://doi.org/10.3390/ijms222011187
Kim, R., Lee, S., Lee, J., et al. (2018). Exosomes derived from microRNA-584 transfected mesenchymal stem cells: Novel alternative therapeutic vehicles for cancer therapy. BMB Reports, 51(8), 406–411. https://doi.org/10.5483/bmbrep.2018.51.8.105
Liu, L., Cheng, M., Zhang, T., et al. (2022). Mesenchymal stem cell-derived extracellular vesicles prevent glioma by blocking M2 polarization of macrophages through a miR-744-5p/TGFB1-dependent mechanism. Cell Biology and Toxicology. https://doi.org/10.1007/s10565-021-09652-7
Zhang, Z., Guo, X., Guo, X., et al. (2021). MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging (Albany NY), 13(4), 5055–5068. https://doi.org/10.18632/aging.202424
Katakowski, M., Buller, B., Zheng, X., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335(1), 201–204. https://doi.org/10.1016/j.canlet.2013.02.019
Lang, F. M., Hossain, A., Gumin, J., et al. (2018). Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-Oncology, 20(3), 380–390. https://doi.org/10.1093/neuonc/nox152
Yan, T., Wu, M., Lv, S., et al. (2021). Exosomes derived from microRNA-512-5p-transfected bone mesenchymal stem cells inhibit glioblastoma progression by targeting JAG1. Aging (Albany NY), 13(7), 9911–9926. https://doi.org/10.18632/aging.202747
Wang, K., Kumar, U. S., Sadeghipour, N., et al. (2021). A microfluidics-based scalable approach to generate extracellular vesicles with enhanced therapeutic MicroRNA loading for intranasal delivery to mouse glioblastomas. ACS Nano. https://doi.org/10.1021/acsnano.1c07587
Rehman, F. U., Liu, Y., Yang, Q., et al. (2022). Heme Oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. Journal of Controlled Release, 345, 696–708. https://doi.org/10.1016/j.jconrel.2022.03.036
Tibensky, M., Jakubechova, J., Altanerova, U., et al. (2022). Gene-directed enzyme/prodrug therapy of rat brain tumor mediated by human mesenchymal stem cell suicide gene extracellular vesicles in vitro and in vivo. Cancers (Basel), 14(3), 735. https://doi.org/10.3390/cancers14030735
Zhu, Q., Ling, X., Yang, Y., et al. (2019). Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Advance Science (Weinh), 6(6), 1801899. https://doi.org/10.1002/advs.201801899
Kong, Y. W., Ferland-McCollough, D., Jackson, T. J., et al. (2012). microRNAs in cancer management. The lancet Oncology, 13(6), e249–e258. https://doi.org/10.1016/s1470-2045(12)70073-6
Yu, L., Gui, S., Liu, Y., et al. (2019). Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging (Albany NY), 11(15), 5300–5318. https://doi.org/10.18632/aging.102092
Altaner, C. (2008). Prodrug cancer gene therapy. Cancer Letters, 270(2), 191–201. https://doi.org/10.1016/j.canlet.2008.04.023
Pastorakova, A., Jakubechova, J., Altanerova, U., et al. (2020). Suicide gene therapy mediated with exosomes produced by mesenchymal stem/stromal cells stably transduced with HSV thymidine kinase. Cancers (Basel), 12(5), 1096. https://doi.org/10.3390/cancers12051096
Webber, J., & Clayton, A. (2013). How pure are your vesicles? Journal of Extracellular Vesicles, 2(1), 19861. https://doi.org/10.3402/jev.v2i0.19861
Lozano-Ramos, I., Bancu, I., Oliveira-Tercero, A., et al. (2015). Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. Journal of Extracellular Vesicles, 4, 27369. https://doi.org/10.3402/jev.v4.27369
Merchant, M. L., Powell, D. W., Wilkey, D. W., et al. (2010). Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics. Clinical Applications, 4(1), 84–96. https://doi.org/10.1002/prca.200800093
Helwa, I., Cai, J. W., Drewry, M. D., et al. (2017). A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. Plos One, 12(1), e0170628. https://doi.org/10.1371/journal.pone.01706285
Iwai, K., Minamisawa, T., Suga, K., et al. (2016). Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. Journal of Extracellular Vesicles, 5, 30829. https://doi.org/10.3402/jev.v5.30829
Li, P., Kaslan, M., Lee, S. H., et al. (2017). Progress in exosome isolation techniques. Theranostics, 7(3), 789–804. https://doi.org/10.7150/thno.18133
Amarnath, S., Foley, J. E., Farthing, D. E., et al. (2015). Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells, 33(4), 1200–1212. https://doi.org/10.1002/stem.1934
Witwer, K. W., Buzas, E. I., Bemis, L. T., et al. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of Extracellular Vesicles, 2,. https://doi.org/10.3402/jev.v2i0.20360
Gheinani, A. H., Vogeli, M., Baumgartner, U., et al. (2018). Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Science and Reports, 8(1), 3945. https://doi.org/10.1038/s41598-018-22142-x
Linares, R., Tan, S., Gounou, C., et al. (2017). Imaging and quantification of extracellular vesicles by transmission electron microscopy. Methods in Molecular Biology, 1545, 43–54. https://doi.org/10.1007/978-1-4939-6728-5_4
Noble, J. M., Roberts, L. M., Vidavsky, N., et al. (2020). Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. Journal of Structural Biology, 210(1), 107474. https://doi.org/10.1016/j.jsb.2020.107474
Hoog, J. L., & Lotvall, J. (2015). Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. Journal of Extracellular Vesicles, 4, 28680. https://doi.org/10.3402/jev.v4.28680
Rikkert, L. G., Nieuwland, R., Terstappen, L. W. M. M., et al. (2019). Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. Journal of Extracellular Vesicles, 8(1), 1555419. https://doi.org/10.1080/20013078.2018.1555419
Bachurski, D., Schuldner, M., Nguyen, P. H., et al. (2019). Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. Journal of Extracellular Vesicles, 8(1), 1596016. https://doi.org/10.1080/20013078.2019.1596016
Arab, T., Mallick, E. R., Huang, Y., et al. (2021). Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. Journal of Extracellular Vesicles, 10(6), e12079. https://doi.org/10.1002/jev2.12079
Kowal, E. J. K., Ter-Ovanesyan, D., Regev, A., et al. (2017). Extracellular vesicle isolation and analysis by western blotting. Methods in Molecular Biology, 1660, 143–152. https://doi.org/10.1007/978-1-4939-7253-1_12
Volgers, C., Benedikter, B. J., Grauls, G. E., et al. (2017). Bead-based flow-cytometry for semi-quantitative analysis of complex membrane vesicle populations released by bacteria and host cells. Microbiological Research, 200, 25–32. https://doi.org/10.1016/j.micres.2017.04.003
Wiklander, O. P. B., Bostancioglu, R. B., Welsh, J. A., et al. (2018). Systematic methodological evaluation of a multiplex bead-based flow cytometry assay for detection of extracellular vesicle surface signatures. Frontiers in Immunology, 9, 1326. https://doi.org/10.3389/fimmu.2018.01326
Kusuma, G. D., Barabadi, M., Tan, J. L., et al. (2018). To protect and to preserve: Novel preservation strategies for extracellular vesicles. Frontiers in Pharmacology, 9, 1199. https://doi.org/10.3389/fphar.2018.01199
Wu, J. Y., Li, Y. J., Hu, X. B., et al. (2021). Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Delivery, 28(1), 162–170. https://doi.org/10.1080/10717544.2020.1869866
Cheng, Y., Zeng, Q., Han, Q., et al. (2019). Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein & Cell, 10(4), 295–299. https://doi.org/10.1007/s13238-018-0529-4
Park, S. J., Jeon, H., Yoo, S.-M., et al. (2018). The effect of storage temperature on the biological activity of extracellular vesicles for the complement system. In Vitro Cellular & Developmental Biology - Animal, 54(6), 423–429. https://doi.org/10.1007/s11626-018-0261-7
Meng, W., He, C., Hao, Y., et al. (2020). Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Delivery, 27(1), 585–598. https://doi.org/10.1080/10717544.2020.1748758
Gaurav, I., Thakur, A., Iyaswamy, A., et al. (2021). Factors affecting extracellular vesicles based drug delivery systems. Molecules. 26(6). https://doi.org/10.3390/molecules26061544
Parfejevs, V., Sagini, K., Buss, A., et al. (2020). Adult stem cell-derived extracellular vesicles in cancer treatment: Opportunities and challenges. Cells, 9(5). https://doi.org/10.3390/cells9051171
Vader, P., Mol, E. A., Pasterkamp, G., et al. (2016). Extracellular vesicles for drug delivery. Advanced Drug Delivery Reviews, 106(Pt A), 148–156. https://doi.org/10.1016/j.addr.2016.02.006
Ruan, S., Zhou, Y., Jiang, X., et al. (2021). Rethinking CRITID procedure of brain targeting drug delivery: Circulation, blood brain barrier recognition, intracellular transport, diseased cell targeting, internalization, and drug release. Advanced Science (Weinh), 8(9), 2004025. https://doi.org/10.1002/advs.202004025
Luan, X., Sansanaphongpricha, K., Myers, I., et al. (2017). Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica, 38(6), 754–763. https://doi.org/10.1038/aps.2017.12
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81774059), the Tianjin Natural Science Foundation (No. 19JCZDJC37100)
Funding
National Natural Science Foundation of China (No. 81774059); Tianjin Natural Science Foundation (No. 19JCZDJC37100).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yuying Guo, Dongsheng Hu and Mingli Li performed the literature search. The first draft of the manuscript was written by Yuying Guo and Dongsheng Hu. Lu Lian, Linna Zhao and Yuying Guo drew the figures. Shixin Xu and Huijing Bao critically revised the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest/Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Y., Hu, D., Lian, L. et al. Stem Cell-derived Extracellular Vesicles: A Promising Nano Delivery Platform to the Brain?. Stem Cell Rev and Rep 19, 285–308 (2023). https://doi.org/10.1007/s12015-022-10455-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10455-4