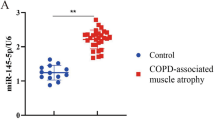
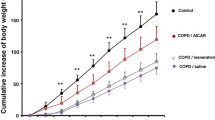
Abstract
Muscle atrophy is a common extrapulmonary co-morbidity affecting about 20% of patients with COPD. However, the mechanism of muscle atrophy in COPD remains unclear. This study investigated the role of the ubiquitin-proteasome system (UPS) and the autophagy system in COPD muscle atrophy and its mechanism. A COPD rat model was established to evaluate the in vitro effects of the UPS and the autophagy system in muscle atrophy. In addition, the role of the UPS, autophagy systems, and the expressions of the PI3K/AKT/FOXO3a pathway were studied in the CSE-induced L6 myoblast cells. Furthermore, we evaluated the effect of FOXO3a in the CSE-induced L6 myoblast cells using siRNA-FOXO3a. The results showed that the expression of ubiquitin-related proteins and autophagy-related proteins were significantly increased in the COPD rat model and CSE-induced L6 myoblast cells. At the same time, there was a concurrent decrease in the phosphorylation protein expression of PI3K and AKT, but the transcriptional activity of FOXO3a was increased in CSE-induced L6 myoblast cells. And siRNA-FOXO3a significantly decreased the expression level of the UPS and the autophagy system in CSE-induced L6 myoblast cells. These results suggest that PI3K/AKT/FOXO3a participates in COPD muscle atrophy by regulating the UPS and the autophagy systems.






Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Henrot, P., Dupin, I., Schilfarth, P., Esteves, P., Blervaque, L., Zysman, M., Gouzi, F., Hayot, M., Pomiès, P., & Berger, P. (2023). Main pathogenic mechanisms and recent advances in COPD peripheral skeletal muscle wasting. International Journal of Molecular Sciences, 24. https://doi.org/10.3390/ijms24076454.
Khalil, R. (2018). Ubiquitin-proteasome pathway and muscle atrophy. Advances in Experimental Medicine and Biology, 1088, 235–248. https://doi.org/10.1007/978-981-13-1435-3_10.
Yin, L., Li, N., Jia, W., Wang, N., Liang, M., Yang, X., & Du, G. (2021). Skeletal muscle atrophy: from mechanisms to treatments. Pharmacological Research, 172, 105807. https://doi.org/10.1016/j.phrs.2021.105807.
Bodine, S., & Baehr, L. (2014). Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. American Journal of Physiology Endocrinology and Metabolism, 307, E469–E484. https://doi.org/10.1152/ajpendo.00204.2014.
Yorimitsu, T., & Klionsky, D. (2005). Autophagy: molecular machinery for self-eating. Cell Death and Differentiation, 1542–1552. https://doi.org/10.1038/sj.cdd.4401765.
Park, E., Choi, H., Truong, C. S., & Jun, H. S. (2023). The inhibition of autophagy and pyroptosis by an ethanol extract of Nelumbo nucifera leaf contributes to the amelioration of dexamethasone-induced muscle atrophy. Nutrients, 15. https://doi.org/10.3390/nu15040804.
Zhang, Y. Y., Gu, L. J., Huang, J., Cai, M. C., Yu, H. L., Zhang, W., Bao, J. F., & Yuan, W. J. (2019). CKD autophagy activation and skeletal muscle atrophy—a preliminary study of mitophagy and inflammation. European Journal of Clinical Nutrition, 73, 950–960. https://doi.org/10.1038/s41430-018-0381-x.
Yang, X., Xue, P., Chen, H., Yuan, M., Kang, Y., Duscher, D., Machens, H., & Chen, Z. (2020). Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis. Theranostics, 10, 1415–1432. https://doi.org/10.7150/thno.40857.
Gu, W., Song, L., Li, X. M., Wang, D., Guo, X. J., & Xu, W. G. (2015). Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Scientific Reports, 5, 8733. https://doi.org/10.1038/srep08733.
Song, J., Liu, J., Cui, C., Hu, H., Zang, N., Yang, M., Yang, J., Zou, Y., Li, J., Wang, L., He, Q., Guo, X., Zhao, R., Yan, F., Liu, F., Hou, X., Sun, Z., & Chen, L. (2023). Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. Journal of Cachexia, Sarcopenia and Muscle, 14, 915–929. https://doi.org/10.1002/jcsm.13177.
Wang, K., Liu, Q., Tang, M., Qi, G., Qiu, C., Huang, Y., Yu, W., Wang, W., Sun, H., Ni, X., Shen, Y., & Fang, X. (2023). Chronic kidney disease-induced muscle atrophy: molecular mechanisms and promising therapies. Biochemical Pharmacology, 208, 115407. https://doi.org/10.1016/j.bcp.2022.115407.
Fang, W., Tseng, Y., Lee, T., Fu, Y., Chang, W., Lo, W., Lin, C., & Lo, Y. (2021). Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. British Journal of Pharmacology, 178, 2998–3016. https://doi.org/10.1111/bph.15472.
Lagirand-Cantaloube, J., Cornille, K., Csibi, A., Batonnet-Pichon, S., Leibovitch, M. P., & Leibovitch, S. A. (2009). Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PloS ONE, 4, e4973. https://doi.org/10.1371/journal.pone.0004973.
Wang, H., Wang, H., Li, X., & Xu, W. (2023). MuRF-1 is involved in laryngeal muscle denervation atrophy by regulating G-actin ubiquitination. The Laryngoscope, https://doi.org/10.1002/lary.31021.
Rodríguez-Arribas, M., Pedro, J. M., Gómez-Sánchez, R., Yakhine-Diop, S. M., Martínez-Chacón, G., Uribe-Carretero, E., De Castro, D. C., Casado-Naranjo, I., López de Munaín, A., Niso-Santano, M., M. Fuentes, J., & A. González-Polo, R. (2016). Pompe disease and autophagy: partners in crime, or cause and consequence? Current Medicinal Chemistry, 23, 2275–2285. https://doi.org/10.2174/1567201812666150122131046.
Nascimbeni, A. C., Fanin, M., Angelini, C., & Sandri, M. (2017). Autophagy dysregulation in Danon disease. Cell Death & Disease, 8, e2565. https://doi.org/10.1038/cddis.2016.475.
Guo, Y., Gosker, H. R., Schols, A. M., Kapchinsky, S., Bourbeau, J., Sandri, M., Jagoe, R. T., Debigaré, R., Maltais, F., Taivassalo, T., & Hussain, S. N. (2013). Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 188, 1313–1320. https://doi.org/10.1164/rccm.201304-0732OC.
Zhao, J., Brault, J. J., Schild, A., Cao, P., Sandri, M., Schiaffino, S., Lecker, S. H., & Goldberg, A. L. (2007). FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metabolism, 6, 472–483. https://doi.org/10.1016/j.cmet.2007.11.004.
Chaanine, A. H., Kohlbrenner, E., Gamb, S. I., Guenzel, A. J., Klaus, K., Fayyaz, A. U., Nair, K. S., Hajjar, R. J., & Redfield, M. M. (2016). FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. American Journal of Physiology Heart and Circulatory Physiology, 311, H1540–H1559. https://doi.org/10.1152/ajpheart.00549.2016.
Geremia, A., Sartori, R., Baraldo, M., Nogara, L., Balmaceda, V., Dumitras, G., Ciciliot, S., Scalabrin, M., Nolte, H., & Blaauw, B. (2022). Activation of Akt-mTORC1 signalling reverts cancer-dependent muscle wasting. Journal of Cachexia, Sarcopenia and Muscle, 13, 648–661. https://doi.org/10.1002/jcsm.12854.
Bodine, S., Stitt, T., Gonzalez, M., Kline, W., Stover, G., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J., Glass, D., & Yancopoulos, G. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology, 3, 1014–1019. https://doi.org/10.1038/ncb1101-1014.
Tzivion, G., Dobson, M., & Ramakrishnan, G. (2011). FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochimica et Biophysica Acta, 1813, 1938–1945. https://doi.org/10.1016/j.bbamcr.2011.06.002.
Stitt, T. N., Drujan, D., Clarke, B. A., Panaro, F., Timofeyva, Y., Kline, W. O., Gonzalez, M., Yancopoulos, G. D., & Glass, D. J. (2004). The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular Cell, 14, 395–403. https://doi.org/10.1016/s1097-2765(04)00211-4.
Gosker, H. R., Kubat, B., Schaart, G., van der Vusse, G. J., Wouters, E. F., & Schols, A. M. (2003). Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. European Respiratory Journal, 22, 280–285. https://doi.org/10.1183/09031936.03.00012803.
Barreiro, E., Ferrer, D., Sanchez, F., Minguella, J., Marin-Corral, J., Martinez-Llorens, J., Lloreta, J., & Gea, J. (2011). Inflammatory cells and apoptosis in respiratory and limb muscles of patients with COPD. Journal of Applied Physiology, 111, 808–817. https://doi.org/10.1152/japplphysiol.01017.2010.
Kovacs, L., & Su, Y. (2017). Redox-dependent calpain signaling in airway and pulmonary vascular remodeling in COPD. Advances in Experimental Medicine and Biology, 967, 139–160. https://doi.org/10.1007/978-3-319-63245-2_9.
Brunnquell, C. R., Vieira, N. A., Sábio, L. R., Sczepanski, F., Cecchini, A. L., Cecchini, R., & Guarnier, F. A. (2015). Oxidative and proteolysis-related parameters of skeletal muscle from hamsters with experimental pulmonary emphysema: a comparison between papain and elastase induction. International Journal of Experimental Pathology, 96, 140–150. https://doi.org/10.1111/iep.12121.
Funding
This research was supported by funds from Shanghai Natural Science Foundation (SNSF) Project (Contract grant number:14ZR1426800).
Author information
Authors and Affiliations
Contributions
H.Y. and G.Z. contribute to the study conduct and draft the manuscript. D.W. and X.H. contribute to data analysis. F.H. contributes to the study design and guide. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, H., Zhu, G., Wang, D. et al. PI3K/AKT/FOXO3a Pathway Induces Muscle Atrophy by Ubiquitin-Proteasome System and Autophagy System in COPD Rat Model. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01232-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01232-w