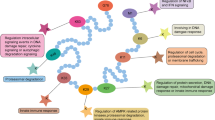
Abstract
F-box proteins are involved in multiple cellular processes through ubiquitylation and consequent degradation of targeted substrates. Any significant mutation in F-box protein-mediated proteolysis can cause human malformations. The various cellular processes F-box proteins involved include cell proliferation, apoptosis, invasion, angiogenesis, and metastasis. To target F-box proteins and their associated signaling pathways for cancer treatment, researchers have developed thousands of F-box inhibitors. The most advanced inhibitor of FBW7, NVD-BK M120, is a powerful P13 kinase inhibitor that has been proven to bring about apoptosis in cancerous human lung cells by disrupting levels of the protein known as MCL1. Moreover, F-box Inhibitors have demonstrated their efficacy for treating certain cancers through targeting particular mutated proteins. This paper explores the key studies on how F-box proteins act and their contribution to malignancy development, which fabricates an in-depth perception of inhibitors targeting the F-box proteins and their signaling pathways that eventually isolate the most promising approach to anti-cancer treatments.




Similar content being viewed by others
References
Bai, C., et al. (1996). SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell, 86(2), 263–274.
Wang, Z., et al. (2014). Roles of F-box proteins in cancer. Nature Reviews Cancer, 14(4), 233–247.
Kipreos, E. T., & Pagano, M. J. G. B. (2000). The F-box protein family. Genome Biology, 1, 1–7.
D’Angiolella, V., et al. (2010). SCF Cyclin F controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature, 466(7302), 138–142.
Duan, S., et al. (2012). FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature, 481(7379), 90–93.
Rye, M., et al. (2011). FBXO11, a regulator of the TGF β pathway, is associated with severe otitis media in Western Australian children. Genes & Immunity, 12(5), 352–359.
Santra, M. K., Wajapeyee, N., & Green, M. R. (2009). F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature, 459(7247), 722–725.
Skaar, J. R., Pagan, J. K., & Pagano, M. (2013). Mechanisms and function of substrate recruitment by F-box proteins. Nature Reviews Molecular cell biology, 14(6), 369–381.
Frescas, D., & Pagano, M. J. N. R. C. (2008). Deregulated proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the scales of cancer. Nature Reviews Cancer, 8(6), 438–449.
Lau, A. W., et al. (2014). The role of FBXW subfamily of F-box proteins in tumorigenesis. 15–45.
Nash, P., et al. (2001). Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature, 414(6863), 514–521.
Orlicky, S., et al. (2003). Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell, 112(2), 243–256.
Lau, A. W., et al. The role of FBXW subfamily of F-box proteins in tumorigenesis, in SCF and APC E3 Ubiquitin Ligases in Tumorigenesis. 2014, Springer. p. 15–45.
Busino, L., et al. (2003). Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature, 426(6962), 87–91.
Jin, J., et al. (2003). SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes & Development, 17(24), 3062–3074.
Watanabe, N., et al. (2004). M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proceedings of the National Academy of Sciences, 101(13), 4419–4424.
Wei, S., et al. (2008). A novel mechanism by which thiazolidinediones facilitate the proteasomal degradation of cyclin D1 in cancer cells. Journal of Biological Chemistry, 283(39), 26759–26770.
Sasajima, H., et al. (2012). Polyubiquitination of the B-cell translocation gene 1 and 2 proteins is promoted by the SCF ubiquitin ligase complex containing βTrCP. Biological and Pharmaceutical Bulletin, 35(9), 1539–1545.
Wojcik, E. J., Glover, D. M., & Hays, T. S. (2000). The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Current Biology, 10(18), 1131–1134.
Zhou, B. P., et al. (2004). Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nature Cell Biology, 6(10), 931–940.
Ray, D., Osmundson, E. C., & Kiyokawa, H. (2006). Constitutive and UV-induced fibronectin degradation is a ubiquitination-dependent process controlled by β-TrCP. Journal of Biological Chemistry, 281(32), 23060–23065.
Ding, Q., et al. (2007). Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Molecular and Cellular Biology, 27(11), 4006–4017.
Dehan, E., et al. (2009). βTrCP-and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Molecular Cell, 33(1), 109–116.
Tan, M., et al. (2006). SAG/ROC-SCFβ-TrCP E3 ubiquitin ligase promotes pro-caspase-3 degradation as a mechanism of apoptosis protection. Neoplasia, 8(12), 1042–1054.
Wang, Z., et al. (2012). Skp2: a novel potential therapeutic target for prostate cancer. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1825(1), 11–17.
Hao, B., et al. (2005) Structural basis of the Cks1-dependent recognition of p27Kip1 by the SCFSkp2 ubiquitin ligase. 20(1): 9–19.
Kamura, T., et al. (2003). Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proceedings of the National Academy of Sciences, 100(18), 10231–10236.
Yu, Z.-K., Gervais, J. L., & Zhang, H. (1998). Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proceedings of the National Academy of Sciences, 95(19), 11324–11329.
Tedesco, D., Lukas, J., & Reed, S. I. (2002). The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein–ubiquitin ligase SCFSkp2. Genes & Development, 16(22), 2946–2957.
Hiramatsu, Y., et al. (2006). Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Research, 66(17), 8477–8483.
Huang, H., et al. (2005). Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proceedings of the National Academy of Sciences, 102(5), 1649–1654.
Seki, R., et al. (2003). Prognostic significance of the F‐box protein Skp2 expression in diffuse large B‐cell lymphoma. American journal of Hematology, 73(4), 230–235.
Wang, H., et al. (2010). Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nature Genetics, 42(1), 83.
Li, J.-Q., et al. (2004). Correlation of Skp2 with carcinogenesis, invasion, metastasis, and prognosis in colorectal tumors. International Journal of Oncology, 25(1), 87–95.
Rose, A. E., et al. (2011). Clinical relevance of SKP2 alterations in metastatic melanoma. Pigment Cell & Melanoma Research, 24(1), 197–206.
Fang, F.-M., et al. (2009). Effect of S-phase kinase-associated protein 2 expression on distant metastasis and survival in nasopharyngeal carcinoma patients. International Journal of Radiation Oncology* Biology* Physics, 73(1), 202–207.
Schüler, S., et al. (2011). SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. International Journal of Oncology, 38(1), 219–225.
Radke, S., Pirkmaier, A., & Germain, D. (2005). Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene, 24(21), 3448–3458.
Ma, X.-M., et al. (2005). Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World Journal of Gastroenterology: WJG, 11(42), 6716.
Wang, Z., et al. (2012). Skp2 is a promising therapeutic target in breast cancer. Frontiers in Oncology, 1, 57.
Kitagawa, M., Lee, S. H., & McCormick F. (2007). Skp2 suppresses p53-dependent apoptosis by inhibiting p300-mediated trans-activation. AACR.
Wu, L., et al. (2012). Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chemistry & Biology, 19(12), 1515–1524.
Nakayama, K., et al. (2004). Skp2-mediated degradation of p27 regulates progression into mitosis. Developmental Cell, 6(5), 661–672.
Chan, C.-H., et al. (2012). The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell, 149(5), 1098–1111.
Hershko, D. D. (2008). Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer, 112(7), 1415–1424.
Lin, H.-K., et al. (2009). Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nature Cell Biology, 11(4), 420–432.
Lin, H.-K., et al. (2010). Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature, 464(7287), 374–379.
Zhang, S., (2011). Computer-aided drug discovery and development. Drug Design and Discovery 23–38.
Chan, C.-H., et al. (2013). Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell, 154(3), 556–568.
Zheng, X.-Y., et al. (2004). Correlation of Skp2 and P27kip1 protein expression and clinicopathological features of prostate cancer. Ai zheng= Aizheng= Chinese Journal of Cancer, 23(2), 215–218.
Schulman, B. A., et al. (2000). Insights into SCF ubiquitin ligases from the structure of the Skp1–Skp2 complex. Nature, 408(6810), 381–386.
Zheng, N., et al. (2002). Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature, 416(6882), 703–709.
Wen, Y., Wang, K., & Yang, K. (2016). Inhibiting the role of Skp2 suppresses cell proliferation and tumorigenesis of human gastric cancer cells via the upregulation of p27kip1. Molecular Medicine Reports, 14(4), 3917–3924.
Lin, S.-X., et al. (2010). Molecular therapy of breast cancer: progress and future directions. Nature Reviews Endocrinology, 6(9), 485–493.
Lu, Y., Zi, X., & Pollak, M. (2004). Molecular mechanisms underlying IGF‐I‐induced attenuation of the growth‐inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. International Journal of Cancer, 108(3), 334–341.
Frankland-Searby, S., & Bhaumik, S. R. (2012). The 26S proteasome complex: an attractive target for cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1825(1), 64–76.
Uddin, S., et al. (2008). Bortezomib (Velcade) induces p27Kip1 expression through S-phase kinase protein 2 degradation in colorectal cancer. Cancer Research, 68(9), 3379–3388.
Mackay, H., et al. (2005). A phase II trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clinical Cancer Research, 11(15), 5526–5533.
Park, E.-J., et al. (2013). Suppression of Src/ERK and GSK-3/β-catenin signaling by pinosylvin inhibits the growth of human colorectal cancer cells. Food and Chemical Toxicology, 55, 424–433.
Voutsadakis, I. A. (2008). The ubiquitin-proteasome system in colorectal cancer. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1782(12), 800–808.
Chen, Q., et al. 441 Krenitsky VP, Xu W, Moutouh-de Parseval L, Webb DR, Mercurio F, Nakayama 442 KI, Nakayama K, Orlowski RZ. Targeting the p27 E3 ligase SCF (Skp2) results in p27-443 and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 111, 4690–4699.
Bochis, O. V., et al. (2015). The role of Skp2 and its substrate CDKN1B (p27) in colorectal cancer. Journal of Gastrointestinal & Liver Diseases, 24(2), 225–234.
Liao, Y., et al. (2014). Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells. Cell Death & Disease, 5(3), e1137–e1137.
Su, J., et al. (2016). Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. American Journal of Cancer Research, 6(9), 1949.
Huang, H.-C., Lin, C.-L., & Lin, J.-K. (2011). 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. Journal of Agricultural and Food Chemistry, 59(12), 6765–6775.
Li, X., et al. (2015). Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model. Oncotarget, 6(39), 41809.
Jiang, W., Lin, M., & Wang, Z. (2020). Dioscin: A new potential inhibitor of Skp2 for cancer therapy. EBioMedicine, 51, 102593.
Lee, H., et al. (2006). Mouse emi1 has an essential function in mitotic progression during early embryogenesis. Molecular and Cellular Biology, 26(14), 5373–5381.
Min, K.-W., et al. (2013). Clear cell carcinomas of the ovary: a multi-institutional study of 129 cases in Korea with prognostic significance of Emi1 and Galectin-3. International Journal Gynecological Pathology, 32(1), 3–14.
Lehman, N. L., et al. (2007). Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors. American Journal Pathology, 170(5), 1793–1805.
Visintin, R., Prinz, S., & Amon, A. (1997). CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science, 278(5337), 460–463.
Sivakumar, S., & Gorbsky, G. J. (2015). Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nature Reviews Molecular Cell Biology, 16(2), 82–94.
Rahimi, H., et al. (2015). The expression pattern of APC2 and APC7 in various cancer cell lines and AML patients. Advances in Medical Sciences, 60(2), 259–263.
VanGenderen, C., Harkness, T. A. A., & Arnason, T. G. (2020). The role of Anaphase Promoting Complex activation, inhibition and substrates in cancer development and progression. Aging (Albany NY), 12(15), 15818.
Machida, Y. J., & Dutta, A. (2007). The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes & Development, 21(2), 184–194.
Sailo, B. L., et al. (2019). FBXW7 in cancer: what has been unraveled thus far? Cancers, 11(2), 246.
Tu, K., et al. (2013). Recombinant human adenovirus-p53 injection induced apoptosis in hepatocellular carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls c-Myc and cyclin E. PloS One, 8(7), e68574.
Sato, M., et al. (2015). MYC is a critical target of FBXW7. Oncotarget, 6(5), 3292.
Wei, W., et al. (2005). The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell, 8(1), 25–33.
Sancho, R., et al. (2013). Fbw7 repression by hes5 creates a feedback loop that modulates Notch-mediated intestinal and neural stem cell fate decisions. PLoS Biology, 11(6), e1001586.
Yokobori, T., et al. (2014). FBXW7 mediates chemotherapeutic sensitivity and prognosis in NSCLCs. Molecular Cancer Research, 12(1), 32–37.
Ren, H., et al. (2013). The PI3 kinase inhibitor NVP-BKM120 induces GSK3/FBXW7-dependent Mcl-1 degradation, contributing to induction of apoptosis and enhancement of TRAIL-induced apoptosis. Cancer Letters, 338(2), 229–238.
Welcker, M., & Clurman, B. E. (2007). Fbw7/hCDC4 dimerization regulates its substrate interactions. Cell Division, 2(1), 1–13.
Tang, X., et al. (2007). Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell, 129(6), 1165–1176.
Flugel, D., Gorlach, A., & Kietzmann T. (2011). Glycogen synthase kinase-3beta regulates cell growth, migration and angiogenesis via Fbw7 and USP-28-dependent degradation of hypoxia-inducible factor-1alpha. Blood.
Cao, J., Ge, M.-H., & Ling Z.-Q. (2016). Fbxw7 tumor suppressor: a vital regulator contributes to human tumorigenesis. Medicine. 95(7).
Isobe, T., et al. (2009). Adenovirus E1A inhibits SCFFbw7 ubiquitin ligase. Journal of Biological Chemistry, 284(41), 27766–27779.
Mao, J.-H., et al. (2004). Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature, 432(7018), 775–779.
Calcagno, D. Q., et al. (2013). MYC, FBXW7 and TP53 copy number variation and expression in gastric cancer. BMC Gastroenterology, 13(1), 1–10.
Kimura, T., et al. (2003). hCDC4b, a regulator of cyclin E, as a direct transcriptional target of p53. Cancer Science, 94(5), 431–436.
Li, Z., et al. (2015). p53 mutation directs AURKA overexpression via miR-25 and FBXW7 in prostatic small cell neuroendocrine carcinoma. Molecular Cancer Research, 13(3), 584–591.
Balamurugan, K., & Sterneck, E. (2013). The many faces of C/EBPδ and their relevance for inflammation and cancer. International Journal of Biological Sciences, 9(9), 917.
Gery, S., et al. (2005). C/EBPδ expression in a BCR-ABL-positive cell line induces growth arrest and myeloid differentiation. Oncogene, 24(9), 1589–1597.
Welcker, M., & Clurman, B. E. (2008). FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature Reviews Cancer, 8(2), 83–93.
Lu, K. P., & Zhou, X. Z. (2007). The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nature Reviews Molecular Cell Biology, 8(11), 904–916.
Min, S.-H., et al. (2012). Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Molecular Cell, 46(6), 771–783.
Wang, L., et al. (2014). Aberrant regulation of FBW7 in cancer. Oncotarget, 5(8), 2000.
Jiang, X., et al. (2012). Numb regulates glioma stem cell fate and growth by altering epidermal growth factor receptor and Skp1-Cullin-F-box ubiquitin ligase activity. Stem Cells, 30(7), 1313–1326.
Wang, Q., et al. (2011). Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen. Oncogene, 30(36), 3875–3886.
Lerner, M., et al. (2011). MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle, 10(13), 2172–2183.
Panka, D. J., et al. (2008). GSK-3β inhibition enhances sorafenib-induced apoptosis in melanoma cell lines. Journal Biological Chemistry, 283(2), 726–732.
Konopleva, M., et al. (2008). Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Research, 68(9), 3413–3420.
Cragg, M. S., et al. (2009). Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nature Reviews Cancer, 9(5), 321–326.
Nijhawan, D., et al. (2003). Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes & Development, 17(12), 1475–1486.
Liu, Y., & Mallampalli, R. K. Small molecule therapeutics targeting F-box proteins in cancer. in Seminars in Cancer Biology. 2016. Elsevier.
Yan, L., et al. (2020). Emerging roles of F-box proteins in cancer drug resistance. Drug Resistance Updates, 49, 100673.
Xiao, Y., et al. (2018). FBXW 7 deletion contributes to lung tumor development and confers resistance to gefitinib therapy. Molecular Oncology, 12(6), 883–895.
Feng, C., Yang, F., & Wang, J. (2017). FBXO4 inhibits lung cancer cell survival by targeting Mcl-1 for degradation. Cancer Gene Therapy, 24(8), 342–347.
Kang, J.-H., et al. (2017). Regulation of FBXO4-mediated ICAM-1 protein stability in metastatic breast cancer. Oncotarget, 8(47), 83100.
Qie, S., et al. (2019). Targeting glutamine-addiction and overcoming CDK4/6 inhibitor resistance in human esophageal squamous cell carcinoma. Nature Communications, 10(1), 1–15.
Lian, Z., et al. (2015). FBXO4 loss facilitates carcinogen induced papilloma development in mice. Cancer Biology & Therapy, 16(5), 750–755.
Yoshida, A., et al. (2019). SLC36A1-mTORC1 signaling drives acquired resistance to CDK4/6 inhibitors. Science Advances, 5(9), eaax6352.
Tekcham, D. S., et al. (2020). F-box proteins and cancer: an update from functional and regulatory mechanism to therapeutic clinical prospects. Theranostics, 10(9), 4150.
Shima, Y., et al. (2008). PML activates transcription by protecting HIPK2 and p300 from SCFFbx3-mediated degradation. Molecular and Cellular Biology, 28(23), 7126–7138.
Chen, B. B., et al. (2013). A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nature Immunology, 14(5), 470–479.
Shao, W., et al. (2016). FBXO3 protein promotes ubiquitylation and transcriptional activity of AIRE (autoimmune regulator). Journal of Biological Chemistry, 291(34), 17953–17963.
Li, D., et al. (2015). F-box protein Fbxo3 targets Smurf1 ubiquitin ligase for ubiquitination and degradation. Biochemical and Biophysical Research Communications, 458(4), 941–945.
Kogure, N., et al. (2017). Low expression of FBXO45 is associated with gastric cancer progression and poor prognosis. Anticancer Research, 37(1), 191–196.
Siepka, S. M., et al. (2007). Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell, 129(5), 1011–1023.
Yamanaka, I., et al. (2007). Presence of robust circadian clock oscillation under constitutive over-expression of mCry1 in rat-1 fibroblasts. FEBS Letters, 581(21), 4098–4102.
Godinho, S. I., et al. (2007). The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science, 316(5826), 897–900.
Busino, L., et al. (2007). SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science, 316(5826), 900–904.
Huber, A.-L., et al. (2016). CRY2 and FBXL3 cooperatively degrade c-MYC. Molecular Cell, 64(4), 774–789.
Correia, S. P., et al. (2019). The circadian E3 ligase complex SCF FBXL3+ CRY targets TLK2. Scientific Reports, 9(1), 1–9.
Fuchs, S. Y., Spiegelman, V. S., & Kumar, K. S. (2004). The many faces of β-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene, 23(11), 2028–2036.
Margottin-Goguet, F., et al. (2003). Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Developmental Cell, 4(6), 813–826.
Wei, X., et al. (2018). SKP2 promotes hepatocellular carcinoma progression through nuclear AMPK-SKP2-CARM1 signaling transcriptionally regulating nutrient-deprived autophagy induction. Cellular Physiology and Biochemistry, 47(6), 2484–2497.
Dorrello, N. V., et al. (2006). S6K1-and ßTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science, 314(5798), 467–471.
Perkins, N. D. (2007). Integrating cell-signalling pathways with NF-κB and IKK function. Nature Reviews Molecular Cell Biology, 8(1), 49–62.
Chen, Z. J. (2005). Ubiquitin signalling in the NF-κB pathway. Nature Cell Biology, 7(8), 758–765.
Yaron, A., et al. (1998). Identification of the receptor component of the IκBα–ubiquitin ligase. Nature, 396(6711), 590–594.
Nakajima, H., et al. (2008). A novel small-molecule inhibitor of NF-κB signaling. Biochemical and Biophysical Research Communications, 368(4), 1007–1013.
Nakayama, K., et al. (2003). Impaired degradation of inhibitory subunit of NF-κB (IκB) and β-catenin as a result of targeted disruption of the β-TrCP1 gene. Proceedings of the National Academy of Sciences, 100(15), 8752–8757.
Yang, H.-S., et al. (2003). The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Molecular and Cellular Biology, 23(1), 26–37.
Blees, J. S., et al. (2012). Erioflorin stabilizes the tumor suppressor Pdcd4 by inhibiting its interaction with the E3-ligase β-TrCP1. PloS one, 7(10), e46567.
Peterson, T. R., et al. (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell, 137(5), 873–886.
Duan, S., et al. (2011). mTOR generates an auto-amplification loop by triggering the βTrCP-and CK1α-dependent degradation of DEPTOR. Molecular cell, 44(2), 317–324.
Wang, Z., et al. (2012). DEPTOR ubiquitination and destruction by SCFβ-TrCP. American Journal of Physiology-Endocrinology and Metabolism, 303(2), E163–E169.
Zhao, Y., Xiong, X., & Sun, Y. (2011). DEPTOR, an mTOR inhibitor, is a physiological substrate of SCFβTrCP E3 ubiquitin ligase and regulates survival and autophagy. Molecular Cell, 44(2), 304–316.
Sakamoto, K. M., et al. (2001). Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proceedings of the National Academy of Sciences, 98(15), 8554–8559.
Sakamoto, K. M., et al. (2003). Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Molecular & Cellular Proteomics, 2(12), 1350–1358.
Schneekloth, A. R., et al. (2008). Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorganic & Medicinal Chemistry Letters, 18(22), 5904–5908.
O’Connor, L., et al. (1998). Bim: a novel member of the Bcl‐2 family that promotes apoptosis. The EMBO Journal, 17(2), 384–395.
Fletcher, J. I., & Huang, D. C. (2008). Controlling the cell death mediators Bax and Bak: puzzles and conundrums. Cell Cycle, 7(1), 39–44.
Rogers, G. C., et al. (2009). The SCFSlimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. Journal of Cell Biology, 184(2), 225–239.
Cunha-Ferreira, I., et al. (2009). The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Current Biology, 19(1), 43–49.
Bornstein, G., et al. (2003). Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. Journal of Biological Chemistry, 278(28), 25752–25757.
Carrano, A. C., et al. (1999). SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biology, 1(4), 193–199.
Bhattacharya, S., et al. (2003). SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene, 22(16), 2443–2451.
Yeh, K.-H., et al. (2001). The F-box protein SKP2 binds to the phosphorylated threonine 380 in cyclin E and regulates ubiquitin-dependent degradation of cyclin E. Biochemical and Biophysical Research Communications, 281(4), 884–890.
Von Der Lehr, N., et al. (2003). The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Molecular Cell, 11(5), 1189–1200.
Yao, F., et al. (2018). SKP2-and OTUD1-regulated non-proteolytic ubiquitination of YAP promotes YAP nuclear localization and activity. Nature Communications, 9(1), 1–16.
Clement, E., et al. (2018). Skp2-dependent reactivation of AKT drives resistance to PI3K inhibitors. Science Signaling, 11(521), eaao3810.
Strohmaier, H., et al. (2001). Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature, 413(6853), 316–322.
Mao, J.-H., et al. (2008). FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science, 321(5895), 1499–1502.
Close, V., et al. (2019). FBXW7 mutations reduce binding of NOTCH1, leading to cleaved NOTCH1 accumulation and target gene activation in CLL. Blood, The Journal of the American Society of Hematology, 133(8), 830–839.
Pine, S. R. (2018). Rethinking Gamma-secretase Inhibitors for Treatment of Non–small-Cell Lung Cancer: Is Notch the Target? Rethinking Gamma Secretase Inhibitors. Clinical Cancer Research, 24(24), 6136–6141.
Gao, J., et al. (2014). Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget, 5(11), 3444.
Babaei-Jadidi, R., et al. (2011). FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. Journal of Experimental Medicine, 208(2), 295–312.
Zhang, Z. & Richmond A. (2021). The role of PI3K inhibition in the treatment of breast cancer, alone or combined with immune checkpoint inhibitors. Frontiers in Molecular Biosciences, 286.
Su, S., et al. (2022). PLK1 inhibition-based combination therapies for cancer management. Translational Oncology, 16, 101332.
Gutteridge, R. E. A., et al. (2016). Plk1 inhibitors in cancer therapy: from laboratory to clinics. Molecular Cancer Therapeutics, 15(7), 1427–1435.
Mori, A., et al. (2018). FBXW 7 modulates malignant potential and cisplatin‐induced apoptosis in cholangiocarcinoma through NOTCH 1 and MCL 1. Cancer Science, 109(12), 3883–3895.
Yada, M., et al. (2004). Phosphorylation‐dependent degradation of c‐Myc is mediated by the F‐box protein Fbw7. The. EMBO Journal, 23(10), 2116–2125.
Gergely, P. A., et al. (2019). Tyrosine kinase inhibitor imatinib mesylate alters DMBA-induced early onco/suppressor gene expression with tissue-specificity in mice. BioMed Research International, 2019, 8670398.
Suenaga, M., et al. (2013). Influence of gefitinib and erlotinib on apoptosis and C-MYC expression in H23 lung cancer cells. Anticancer Research, 33(4), 1547–1554.
Lee, E. K., et al. (2013). The FBXO4 tumor suppressor functions as a barrier to BRAFV600E-dependent metastatic melanoma. Molecular and Cellular Biology, 33(22), 4422–4433.
Achkar, I. W., et al. (2018). Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. Journal of Translational Medicine, 16(1), 1–12.
Roy, S., et al. (2018). p38 MAPK pathway and its interaction with TRF2 in cisplatin induced chemotherapeutic response in head and neck cancer. Oncogenesis, 7(7), 1–16.
Liu, K., et al. (2013). Synergistic effect of paclitaxel and epigenetic agent phenethyl isothiocyanate on growth inhibition, cell cycle arrest and apoptosis in breast cancer cells. Cancer Cell International, 13(1), 1–8.
Qie, S., et al. (2017). Fbxo4-mediated degradation of Fxr1 suppresses tumorigenesis in head and neck squamous cell carcinoma. Nat Commun, 8, 1534.
Wang, B., et al. (2021). circNRIP1 facilitates keloid progression via FXR1-mediated upregulation of miR-503-3p and miR-503-5p. International Journal of Molecular Medicine, 47(5), 1–13.
Peng, J., et al. (2018). An Hsp20-FBXO4 axis regulates adipocyte function through modulating PPARγ ubiquitination. Cell Reports, 23(12), 3607–3620.
Lin, D. I., et al. (2006). Phosphorylation-dependent ubiquitination of cyclin D1 by the SCFFBX4-αB crystallin complex. Molecular Cell, 24(3), 355–366.
Braal, C. L., et al. (2021). Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs, 81(3), 317–331.
Andrzejewski, S., et al. (2017). PGC-1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metabolism, 26(5), 778–787.e5.
Qie, S., et al. (2019). Targeting glutamine-addiction and overcoming CDK4/6 inhibitor resistance in human esophageal squamous cell carcinoma. Nature Communications, 10(1), 1296.
D’Angiolella, V., et al. (2012). Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell, 149(5), 1023–1034.
Fu, J., et al. (2013). Low cyclin F expression in hepatocellular carcinoma associates with poor differentiation and unfavorable prognosis. Cancer Science, 104(4), 508–515.
Emanuele, M. J., et al. (2011). Global identification of modular cullin-RING ligase substrates. Cell, 147(2), 459–474.
Walter, D., et al. (2016). SCF (cyclin F)-dependent degradation of CDC6 suppresses DNA re-replication. Nat Commun, 7, 10530.
Altomonte, J., et al. (2003). Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. American Journal of Physiology Endocrinology and Metabolism, 285(4), E718–E728.
Haeusler, R. A., Kaestner, K. H., & Accili, D. J. J. O. B. C. (2010). FoxOs function synergistically to promote glucose production. Journal of Biologcal Chemistry, 285(46), 35245–35248.
Langlet, F., et al. (2017). Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell, 171(4), 824–835.e18.
Pandey, A., et al. (2016). FoxO1 inhibitors: the future medicine for metabolic disorders? Current Diabetes Reviews, 12(3), 223–230.
Mallampalli, R. K., et al. (2013). Targeting F box protein Fbxo3 to control cytokine-driven inflammation. Journal Immunology, 191(10), 5247–5255.
Lomonosov, M., et al. (2011). Expression of Fbxo7 in haematopoietic progenitor cells cooperates with p53 loss to promote lymphomagenesis. PLoS One, 6(6), e21165.
Zheng, H., et al. (2014). PKD1 phosphorylation-dependent degradation of SNAIL by SCF-FBXO11 regulates epithelial-mesenchymal transition and metastasis. Cancer cell, 26(3), 358–373.
Pighi, C., et al. (2021). Frequent mutations of FBXO11 highlight BCL6 as a therapeutic target in Burkitt lymphoma. Blood Advances, 5(23), 5239–5257.
Zou, S., et al. (2018). FBXO31 suppresses gastric cancer EMT by targeting Snail1 for proteasomal degradationubiquitination and degradation of Snail1 by FBXO31. Molecular Cancer Research, 16(2), 286–295.
Malonia, S. K., et al. (2015). F-box protein FBXO31 directs degradation of MDM2 to facilitate p53-mediated growth arrest following genotoxic stress. Proceedings of the National Academy of Sciences, 112(28), 8632–8637.
Liu, J., et al. (2014). F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6). Journal of Biological Chemistry, 289(31), 21508–21518.
Chung, F.-Z., et al. (2014). Fbxo45 inhibits calcium-sensitive proteolysis of N-cadherin and promotes neuronal differentiation. Journal of Biological Chemistry, 289(41), 28448–28459.
Chen, X., et al. (2014). Fbxo45-mediated degradation of the tumor-suppressor Par-4 regulates cancer cell survival. Cell Death & Differentiation, 21(10), 1535–1545.
Peschiaroli, A., et al. (2009). The F-box protein FBXO45 promotes the proteasome-dependent degradation of p73. Oncogene, 28(35), 3157–3166.
English, J. M., & Cobb, M. H. J. T. I. P. S. (2002). Pharmacological inhibitors of MAPK pathways. Trends in Pharmacological Sciences, 23(1), 40–45.
Sahasrabuddhe, A. A., et al. (2021). A novel FBXO45-Gef-H1 axis controls oncogenic signaling in B-cell lymphoma. Blood, 138(Supplement 1), 711–711.
Correia, S., et al. (2019). The circadian E3 ligase complex SCF (FBXL3+ CRY) targets TLK2. Sci Rep, 9(1), 198.
Hart, M., et al. (1999). The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Current Biology, 9(4), 207–211.
Wei, S., et al. (2007). Thiazolidinediones modulate the expression of β-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor γ. Molecular Pharmacology, 72(3), 725–733.
Winston, J. T., et al. (1999). The SCFβ-TRCP–ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes & Development, 13(3), 270–283.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant Numbers. 31900913, 82020108030, and U21A20416), the National Key Research Program (grant number 2018YFE0195100), and the Postgraduate Education Reform Project of Zhengzhou university in 2021 (grant number YJSJY202159). This work was also supported by the Education Reform Research Project (for international students) of Zhengzhou University in 2022 (grant number 2022ZZUJGXMLXS-031), the Training Program of Innovation and Entrepreneurship for College Students (Undergraduate Research Training Program number 202310459149). Thanks to the support from the National Supercomputing Center in Zhengzhou, thanks to the support from Shanghai Synchrotron Radiation Facility and National Facility for Protein Science Shanghai(NFPSS).
Funding
The relevant information of the fund is in the ‘Acknowledgements’ section.
Author information
Authors and Affiliations
Contributions
C.Z., Y.N. and J.D. contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naseem, Y., Zhang, C., Zhou, X. et al. Inhibitors Targeting the F-BOX Proteins. Cell Biochem Biophys 81, 577–597 (2023). https://doi.org/10.1007/s12013-023-01160-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01160-1