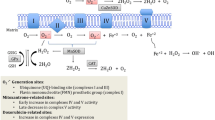
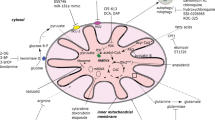
Abstract
The advent of BCR-ABL tyrosine kinase inhibitors (TKIs) targeted therapy revolutionized the treatment of chronic myeloid leukemia (CML) patients. Mitochondria are the key organelles for the maintenance of myocardial tissue homeostasis. However, cardiotoxicity associated with BCR-ABL1 TKIs can directly or indirectly cause mitochondrial damage and dysfunction, playing a pivotal role in cardiomyocytes homeostatic system and putting the cancer survivors at higher risk. In this review, we summarize the cardiotoxicity caused by BCR-ABL1 TKIs and the underlying mechanisms, which contribute dominantly to the damage of mitochondrial structure and dysfunction: endoplasmic reticulum (ER) stress, mitochondrial stress, damage of myocardial cell mitochondrial respiratory chain, increased production of mitochondrial reactive oxygen species (ROS), and other kinases and other potential mechanisms of cardiotoxicity induced by BCR-ABL1 TKIs. Furthermore, detection and management of BCR-ABL1 TKIs will promote our rational use, and cardioprotection strategies based on mitochondria will improve our understanding of the cardiotoxicity from a mitochondrial perspective. Ultimately, we hope shed light on clinical decision-making. By integrate and learn from both research and practice, we will endeavor to minimize the mitochondria-mediated cardiotoxicity and reduce the adverse sequelae associated with BCR-ABL1 TKIs.



Similar content being viewed by others
Data availability
Data availability is not applicable to this review as no new data were created in this study.
References
Jabbour, E., & Kantarjian, H. (2020). Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. American Journal of Hematology, 95(6), 691–709. https://doi.org/10.1002/ajh.25792
Faderl, S., Talpaz, M., Estrov, Z., O’Brien, S., Kurzrock, R., & Kantarjian, H. M. (1999). The biology of chronic myeloid Leukemia. The New England Journal of Medicine, 341(3), 164–172. https://doi.org/10.1056/nejm199907153410306
Iacoboni, S. J., Plunkett, W., Kantarjian, H. M., Estey, E., Keating, M. J., McCredie, K. B., & Freireich, E. J. (1986). High-dose cytosine arabinoside: Treatment and cellular pharmacology of chronic myelogenous Leukemia blast crisis. Journal of clinical oncology : Official journal of the American Society of Clinical Oncology, 4(7), 1079–1088. https://doi.org/10.1200/jco.1986.4.7.1079
Esteban-Villarrubia, J., Soto-Castillo, J. J., Pozas, J., San Roman-Gil, M., Orejana-Martin, I., Torres-Jimenez, J., Carrato, A., Alonso-Gordoa, T., & Molina-Cerrillo, J. (2020). Tyrosine kinase receptors in oncology. International Journal of Molecular Sciences, 21(22), 48. https://doi.org/10.3390/ijms21228529
Braun, T. P., Eide, C. A., & Druker, B. J. (2020). Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer cell, 37(4), 530–542. https://doi.org/10.1016/j.ccell.2020.03.006
Hehlmann, R., Müller, M. C., Lauseker, M., Hanfstein, B., Fabarius, A., Schreiber, A., Proetel, U., Pletsch, N., Pfirrmann, M., Haferlach, C., Schnittger, S., Einsele, H., Dengler, J., Falge, C., Kanz, L., Neubauer, A., Kneba, M., Stegelmann, F., Pfreundschuh, M., … Hochhaus, A. (2014). Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: Results from the randomized CML-study IV. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 32(5), 415–423. https://doi.org/10.1200/jco.2013.49.9020
Cortes, J. E., Saglio, G., Kantarjian, H. M., Baccarani, M., Mayer, J., Boqué, C., Shah, N. P., Chuah, C., Casanova, L., Bradley-Garelik, B., Manos, G., & Hochhaus, A. (2016). Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 34(20), 2333–2340. https://doi.org/10.1200/JCO.2015.64.8899
Bhamidipati, P. K., Kantarjian, H., Cortes, J., Cornelison, A. M., & Jabbour, E. (2013). Management of imatinib-resistant patients with chronic myeloid leukemia. Therapeutic Advances in Hematology, 4(2), 103–117. https://doi.org/10.1177/2040620712468289
Kamath, A. V., Wang, J., Lee, F. Y., & Marathe, P. H. (2008). Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): A potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemotherapy and Pharmacology, 61(3), 365–376.
Reinwald, M., Schleyer, E., Kiewe, P., Blau, I. W., Burmeister, T., Pursche, S., Neumann, M., Notter, M., Thiel, E., Hofmann, W.-K., Kolb, H.-J., Burdach, S., & Bender, H.-U. (2014). Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive Leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. BioMed Research International. https://doi.org/10.1155/2014/637059
Hoy, S. M. (2014). Ponatinib: A review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic Leukaemia. Drugs, 74(7), 793–806. https://doi.org/10.1007/s40265-014-0216-6
Gunnarsson, N., Sandin, F., Höglund, M., Stenke, L., Björkholm, M., Lambe, M., Olsson-Strömberg, U., Richter, J., & Själander, A. (2016). Population-based assessment of chronic myeloid leukemia in Sweden: Striking increase in survival and prevalence. European Journal of Haematology, 97(4), 387–392. https://doi.org/10.1111/ejh.12743
Saussele, S., Krauss, M. P., Hehlmann, R., Lauseker, M., Proetel, U., Kalmanti, L., Hanfstein, B., Fabarius, A., Kraemer, D., Berdel, W. E., Bentz, M., Staib, P., de Wit, M., Wernli, M., Zettl, F., Hebart, H. F., Hahn, M., Heymanns, J., Schmidt-Wolf, I., … Müller, M. C. (2015). Impact of comorbidities on overall survival in patients with chronic myeloid Leukemia: Results of the randomized CML study IV. Blood, 126(1), 42–49. https://doi.org/10.1182/blood-2015-01-617993
Sanz, A., Ayala, R., Hernández, G., Lopez, N., Gil-Alos, D., Gil, R., Colmenares, R., Carreño-Tarragona, G., Sánchez-Pina, J., Alonso, R. A., García-Barrio, N., Pérez-Rey, D., Meloni, L., Calbacho, M., Cruz-Rojo, J., Pedrera-Jiménez, M., Serrano-Balazote, P., de la Cruz, J., & Martínez-López, J. (2022). Outcomes and patterns of treatment in chronic myeloid leukemia, a global perspective based on a real-world data global network. Blood Cancer Journal, 12(6), 94. https://doi.org/10.1038/s41408-022-00692-8
Tajiri, K., Aonuma, K., & Sekine, I. (2017). Cardiovascular toxic effects of targeted cancer therapy. Japanese Journal of Clinical Oncology, 47(9), 779–785. https://doi.org/10.1093/jjco/hyx071
Moslehi, J. J., & Deininger, M. (2015). Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid Leukemia. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 33(35), 4210–4218. https://doi.org/10.1200/jco.2015.62.4718
Wells, Q. S., & Lenihan, D. J. (2010). Reversibility of left ventricular dysfunction resulting from chemotherapy: Can this be expected? Progress in Cardiovascular Diseases, 53(2), 140–148. https://doi.org/10.1016/j.pcad.2010.06.005
Maharsy, W., Aries, A., Mansour, O., Komati, H., & Nemer, M. (2014). Ageing is a risk factor in imatinib mesylate cardiotoxicity. European Journal of Heart Failure, 16(4), 367–376. https://doi.org/10.1002/ejhf.58
Gugliotta, G., Castagnetti, F., Breccia, M., Levato, L., D’Adda, M., Stagno, F., Tiribelli, M., Salvucci, M., Fava, C., Martino, B., Cedrone, M., Bocchia, M., Trabacchi, E., Cavazzini, F., Usala, E., Russo Rossi, A., Bochicchio, M. T., Soverini, S., Alimena, G., … Rosti, G. (2015). Long-term outcome of a phase 2 trial with nilotinib 400 mg twice daily in first-line treatment of chronic myeloid leukemia. Haematologica, 100(9), 1146–1150. https://doi.org/10.3324/haematol.2015.129221
Ran, H. H., Zhang, R., Lu, X. C., Yang, B., Fan, H., & Zhu, H. L. (2012). Imatinib-induced decompensated heart failure in an elderly patient with chronic myeloid leukemia: Case report and literature review. Journal of Geriatric Cardiology: JGC, 9(4), 411–414. https://doi.org/10.3724/sp.J.1263.2012.05251
Hoffmann, V. S., Baccarani, M., Hasford, J., Castagnetti, F., Di Raimondo, F., Casado, L. F., Turkina, A., Zackova, D., Ossenkoppele, G., Zaritskey, A., Höglund, M., Simonsson, B., Indrak, K., Sninska, Z., Sacha, T., Clark, R., Bogdanovic, A., Hellmann, A., Griskevicius, L., … Hehlmann, R. (2017). Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia, 31(3), 593–601. https://doi.org/10.1038/leu.2016.246
Berman, E. (2022). How I treat chronic-phase chronic myelogenous Leukemia. Blood, 139(21), 3138–3147. https://doi.org/10.1182/blood.2021011722
Gustafson, D., Fish, J. E., Lipton, J. H., & Aghel, N. (2020). Mechanisms of cardiovascular toxicity of BCR-ABL1 Tyrosine kinase inhibitors in chronic myelogenous Leukemia. Current Hematologic Malignancy Reports, 15(1), 20–30. https://doi.org/10.1007/s11899-020-00560-x
Osellame, L. D., Blacker, T. S., & Duchen, M. R. (2012). Cellular and molecular mechanisms of mitochondrial function. Best Practice & Research Clinical endocrinology & metabolism, 26(6), 711–723. https://doi.org/10.1016/j.beem.2012.05.003
Kerkelä, R., Grazette, L., Yacobi, R., Iliescu, C., Patten, R., Beahm, C., Walters, B., Shevtsov, S., Pesant, S., Clubb, F. J., Rosenzweig, A., Salomon, R. N., Van Etten, R. A., Alroy, J., Durand, J. B., & Force, T. (2006). Cardiotoxicity of the Cancer Therapeutic Agent Imatinib Mesylate. Nature medicine, 12(8), 908–916. https://doi.org/10.1038/nm1446
Will, Y., Dykens, J. A., Nadanaciva, S., Hirakawa, B., Jamieson, J., Marroquin, L. D., Hynes, J., Patyna, S., & Jessen, B. A. (2008). Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicological Sciences : An Official Journal of the Society of Toxicology, 106(1), 153–161. https://doi.org/10.1093/toxsci/kfn157
Varga, Z. V., Ferdinandy, P., Liaudet, L., & Pacher, P. (2015). Drug-induced mitochondrial dysfunction and cardiotoxicity. American Journal of Physiology Heart and Circulatory Physiology, 309(9), H1453-1467. https://doi.org/10.1152/ajpheart.00554.2015
Murphy, E., Ardehali, H., Balaban, R. S., DiLisa, F., Dorn, G. W., 2nd., Kitsis, R. N., Otsu, K., Ping, P., Rizzuto, R., Sack, M. N., Wallace, D., & Youle, R. J. (2016). Mitochondrial function, biology, and role in disease: A scientific statement from the american heart association. Circulation Research, 118(12), 1960–1991. https://doi.org/10.1161/res.0000000000000104
Nunnari, J., & Suomalainen, A. (2012). Mitochondria: In sickness and in health. Cell, 148(6), 1145–1159. https://doi.org/10.1016/j.cell.2012.02.035
Shadel, G. S., & Horvath, T. L. (2015). Mitochondrial ROS signaling in organismal homeostasis. Cell, 163(3), 560–569. https://doi.org/10.1016/j.cell.2015.10.001
Nguyen, B. Y., Ruiz-Velasco, A., Bui, T., Collins, L., Wang, X., & Liu, W. (2019). Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. British Journal of Pharmacology, 176(22), 4302–4318. https://doi.org/10.1111/bph.14431
Pouwer, M. G., Pieterman, E. J., Verschuren, L., Caspers, M. P. M., Kluft, C., Garcia, R. A., Aman, J., Jukema, J. W., & Princen, H. M. G. (2018). The BCR-ABL1 inhibitors imatinib and ponatinib decrease plasma cholesterol and atherosclerosis, and nilotinib and ponatinib activate coagulation in a translational mouse model. Frontiers in Cardiovascular Medicine. https://doi.org/10.3389/fcvm.2018.00055
Tang, X., Wang, Z., Hu, S., & Zhou, B. (2022). Assessing Drug-induced mitochondrial toxicity in cardiomyocytes: Implications for preclinical cardiac safety evaluation. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14071313
Wang, W., Fernandez-Sanz, C., & Sheu, S. S. (2018). Regulation of mitochondrial bioenergetics by the non-canonical roles of mitochondrial dynamics proteins in the heart. Biochimica et Biophysica Acta Molecular Basis of Disease, 1864(5 Pt B), 1991–2001. https://doi.org/10.1016/j.bbadis.2017.09.004
Shoshan-Barmatz, V., De Pinto, V., Zweckstetter, M., Raviv, Z., Keinan, N., & Arbel, N. (2010). VDAC, a multi-functional mitochondrial protein regulating cell life and death. Molecular Aspects of Medicine, 31(3), 227–285. https://doi.org/10.1016/j.mam.2010.03.002
Vercellino, I., & Sazanov, L. A. (2022). The assembly, regulation and function of the mitochondrial respiratory chain. Nature Reviews Molecular Cell Biology, 23(2), 141–161. https://doi.org/10.1038/s41580-021-00415-0
Vogel, F., Bornhövd, C., Neupert, W., & Reichert, A. S. (2006). Dynamic subcompartmentalization of the mitochondrial inner membrane. The Journal of cell biology, 175(2), 237–247. https://doi.org/10.1083/jcb.200605138
Cao, Y. P., & Zheng, M. (2019). Mitochondrial dynamics and inter-mitochondrial communication in the heart. Archives of Biochemistry and Biophysics. https://doi.org/10.1016/j.abb.2019.01.017
Yin, Y., & Shen, H. (2021). Advances in cardiotoxicity induced by altered mitochondrial dynamics and mitophagy. Frontiers in Cardiovascular Medicine, 8, 739095. https://doi.org/10.3389/fcvm.2021.739095
Sack, M. N., Fyhrquist, F. Y., Saijonmaa, O. J., Fuster, V., & Kovacic, J. C. (2017). Basic biology of oxidative stress and the cardiovascular system: Part 1 of a 3-Part Series. Journal of the American College of Cardiology, 70(2), 196–211. https://doi.org/10.1016/j.jacc.2017.05.034
Lauritzen, K. H., Kleppa, L., Aronsen, J. M., Eide, L., Carlsen, H., Haugen, Ø. P., Sjaastad, I., Klungland, A., Rasmussen, L. J., Attramadal, H., Storm-Mathisen, J., & Bergersen, L. H. (2015). Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. American journal of physiology Heart and circulatory physiology, 309(3), 434–449. https://doi.org/10.1152/ajpheart.00253.2014
Liu, M., & Wu, Y. (2022). Role of mitophagy in coronary heart Disease: Targeting the mitochondrial dysfunction and inflammatory regulation. Frontiers in Cardiovascular Medicine, 9, 819454. https://doi.org/10.3389/fcvm.2022.819454
Zhou, B., & Tian, R. (2018). Mitochondrial dysfunction in pathophysiology of heart failure. The Journal of clinical Investigation, 128(9), 3716–3726. https://doi.org/10.1172/jci120849
Zamorano, J. L., Lancellotti, P., Rodriguez Muñoz, D., Aboyans, V., Asteggiano, R., Galderisi, M., Habib, G., Lenihan, D. J., Lip, G. Y., Lyon, A. R., Lopez Fernandez, T., Mohty, D., Piepoli, M. F., Tamargo, J., Torbicki, A., Suter, T. M., Zamorano, J. L., Aboyans, V., Achenbach, S., … Windecker, S. (2017). 2016 ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: The task force for cancer treatments and cardiovascular toxicity of the european society of cardiology (ESC). European Journal of Heart Failure, 19(1), 9–42. https://doi.org/10.1002/ejhf.654
Force, T., Krause, D. S., & Van Etten, R. A. (2007). Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature Reviews Cancer, 7(5), 332–344. https://doi.org/10.1038/nrc2106
Liu, Y., Huang, Y., Xu, C., An, P., Luo, Y., Jiao, L., Luo, J., & Li, Y. (2022). Mitochondrial dysfunction and therapeutic perspectives in cardiovascular diseases. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms232416053
Waseem, M., & Parvez, S. (2013). Mitochondrial dysfunction mediated cisplatin induced toxicity: Modulatory role of curcumin. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 53, 334–342. https://doi.org/10.1016/j.fct.2012.11.055
Gorini, S., De Angelis, A., Berrino, L., Malara, N., Rosano, G., & Ferraro, E. (2018). Chemotherapeutic drugs and mitochondrial dysfunction: Focus on doxorubicin, trastuzumab, and sunitinib. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2018/7582730
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., & Gianni, L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews, 56(2), 185–229. https://doi.org/10.1124/pr.56.2.6
Wu, D., Wang, X., & Sun, H. (2018). The role of mitochondria in cellular toxicity as a potential drug target. Cell Biology and Toxicology, 34(2), 87–91. https://doi.org/10.1007/s10565-018-9425-1
Kim, C. W., & Choi, K. C. (2021). Effects of anticancer drugs on the cardiac mitochondrial toxicity and their underlying mechanisms for novel cardiac protective strategies. Life Sciences, 277, 119607. https://doi.org/10.1016/j.lfs.2021.119607
Lushchak, V. I. (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biological Interactions, 224, 164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Holmström, K. M., & Finkel, T. (2014). Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature Reviews Molecular cell biology, 15(6), 411–421. https://doi.org/10.1038/nrm3801
Fu, Z., Guo, J., Jing, L., Li, R., Zhang, T., & Peng, S. (2010). Enhanced toxicity and ROS generation by doxorubicin in primary cultures of cardiomyocytes from neonatal metallothionein-I/II null mice. Toxicology in vitro : An International Journal Published in Association with BIBRA, 24(6), 1584–1591. https://doi.org/10.1016/j.tiv.2010.06.009
Tocchetti, C. G., Molinaro, M., Angelone, T., Lionetti, V., Madonna, R., Mangiacapra, F., Moccia, F., Penna, C., Sartiani, L., Quaini, F., & Pagliaro, P. (2015). Nitroso-redox balance and modulation of basal myocardial function: An update from the italian society of cardiovascular research (SIRC). Current Drug Targets, 16(8), 895–903. https://doi.org/10.2174/1389450116666150304103517
Patergnani, S., Suski, J. M., Agnoletto, C., Bononi, A., Bonora, M., De Marchi, E., Giorgi, C., Marchi, S., Missiroli, S., Poletti, F., Rimessi, A., Duszynski, J., Wieckowski, M. R., & Pinton, P. (2011). Calcium signaling around mitochondria associated membranes (MAMs). Cell Communication and Signaling : CCS,919. https://doi.org/10.1186/1478-811x-9-19
Olson, M. L., Chalmers, S., & McCarron, J. G. (2012). Mitochondrial organization and Ca2+ uptake. Biochemical Society Transactions, 40(1), 158–167. https://doi.org/10.1042/bst20110705
Zhang, Z., Zhao, L., Zhou, Y., Lu, X., Wang, Z., Wang, J., & Li, W. (2017). Taurine ameliorated homocysteine-induced H9C2 cardiomyocyte apoptosis by modulating endoplasmic reticulum stress. Apoptosis : An International Journal on Programmed Cell Death, 22(5), 647–661. https://doi.org/10.1007/s10495-017-1351-9
Marcolino, M. S., Boersma, E., Clementino, N. C., Nunes, M. D., Barbosa, M. M., Silva, M. H., Geleijnse, M. L., & Ribeiro, A. L. (2011). The duration of the use of imatinib mesylate is only weakly related to elevated BNP levels in chronic myeloid Leukaemia patients. Hematological Oncology, 29(3), 124–130. https://doi.org/10.1002/hon.967
Turrisi, G., Montagnani, F., Grotti, S., Marinozzi, C., Bolognese, L., & Fiorentini, G. (2010). Congestive heart failure during imatinib mesylate treatment. International Journal of Cardiology, 145(1), 148–150. https://doi.org/10.1016/j.ijcard.2009.07.006
Rousselot, P., Cony-Makhoul, P., Nicolini, F., Mahon, F. X., Berthou, C., Réa, D., Reiffers, J., Bornand, A., Saint-Jean, O., Guilhot, J., & Guilhot, F. (2013). Long-term safety and efficacy of imatinib mesylate (Gleevec®) in elderly patients with chronic phase chronic myelogenous leukemia: Results of the AFR04 study. American Journal of Hematology, 88(1), 1–4. https://doi.org/10.1002/ajh.23330
Toubert, M. E., Vercellino, L., Faugeron, I., Lussato, D., Hindie, E., & Bousquet, G. (2011). Fatal heart failure after a 26-month combination of tyrosine kinase inhibitors in a papillary thyroid cancer. Thyroid: Official Journal of the American Thyroid Association, 21(4), 451–454. https://doi.org/10.1089/thy.2010.0270
Bouitbir, J., Panajatovic, M. V., Frechard, T., Roos, N. J., & Krähenbühl, S. (2020). Imatinib and Dasatinib Provoke Mitochondrial Dysfunction Leading to Oxidative Stress in C2C12 Myotubes and Human RD Cells. Front Pharmacol, 11, 1106. https://doi.org/10.3389/fphar.2020.01106
Bouitbir, J., Panajatovic, M. V., & Krähenbühl, S. (2022). Mitochondrial Toxicity Associated with Imatinib and Sorafenib in Isolated Rat Heart Fibers and the Cardiomyoblast H9c2 Cell Line. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms23042282
Slee, E. A., Adrain, C., & Martin, S. J. (2001). Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. The Journal of Biological Chemistry, 276(10), 7320–7326. https://doi.org/10.1074/jbc.M008363200
Joza, N., Susin, S. A., Daugas, E., Stanford, W. L., Cho, S. K., Li, C. Y., Sasaki, T., Elia, A. J., Cheng, H. Y., Ravagnan, L., Ferri, K. F., Zamzami, N., Wakeham, A., Hakem, R., Yoshida, H., Kong, Y. Y., Mak, T. W., Zúñiga-Pflücker, J. C., Kroemer, G., & Penninger, J. M. (2001). Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature, 410(6828), 549–554. https://doi.org/10.1038/35069004
Finsterer, J., & Ohnsorge, P. (2013). Influence of mitochondrion-toxic agents on the cardiovascular system. Regulatory Toxicology and Pharmacology: RTP, 67(3), 434–445. https://doi.org/10.1016/j.yrtph.2013.09.002
Yu, C., Krystal, G., Varticovksi, L., McKinstry, R., Rahmani, M., Dent, P., & Grant, S. (2002). Pharmacologic mitogen-activated protein/extracellular signal-regulated kinase kinase/mitogen-activated protein kinase inhibitors interact synergistically with STI571 to induce apoptosis in Bcr/Abl-expressing human Leukemia cells. Cancer Research, 62(1), 188–199.
Thomson, M. (2002). Evidence of undiscovered cell regulatory mechanisms: Phosphoproteins and protein kinases in mitochondria. Cellular and Molecular Life Sciences : CMLS, 59(2), 213–219. https://doi.org/10.1007/s00018-002-8417-7
McMullen, C. J., Chalmers, S., Wood, R., Cunningham, M. R., & Currie, S. (2020). Sunitinib and Imatinib display differential cardiotoxicity in adult rat cardiac fibroblasts that involves a role for calcium/calmodulin dependent protein kinase II. Frontiers in Cardiovascular Medicine, 7, 630480. https://doi.org/10.3389/fcvm.2020.630480
Song, C., Li, D., Zhang, J., & Zhao, X. (2022). Role of ferroptosis in promoting cardiotoxicity induced by Imatinib Mesylate via down-regulating Nrf2 pathways in vitro and in vivo. Toxicology and Applied Pharmacology, 435, 115852. https://doi.org/10.1016/j.taap.2021.115852
Singh, A. P., Umbarkar, P., Tousif, S., & Lal, H. (2020). Cardiotoxicity of the BCR-ABL1 tyrosine kinase inhibitors: Emphasis on ponatinib. International Journal of Cardiology, 316, 214–221. https://doi.org/10.1016/j.ijcard.2020.05.077
Makeeva, L. M., Emelina, E. I., Gendlin, G. E., Nikitin, I. G., Vasyuk, Y. A., & Nesvetov, V. V. (2017). Pulmonary arterial hypertension and chronic heart failure as dasatinib cardiotoxicity. A case report. Kardiologiia, 57(S4), 53–60.
Motokawa, T., Ikeda, S., Ueno, Y., Eguchi, M., Minami, T., Kawano, H., Kobayashi, K., Imaizumi, Y., & Maemura, K. (2022). Comparison of dasatinib- and imatinib-related cardiotoxic adverse events in Japanese patients with chronic myeloid Leukemia and gastrointestinal stromal tumor. Circulation Reports, 4(1), 1–8. https://doi.org/10.1253/circrep.CR-21-0140
Guignabert, C., Phan, C., Seferian, A., Huertas, A., Tu, L., Thuillet, R., Sattler, C., Le Hiress, M., Tamura, Y., Jutant, E. M., Chaumais, M. C., Bouchet, S., Manéglier, B., Molimard, M., Rousselot, P., Sitbon, O., Simonneau, G., Montani, D., & Humbert, M. (2016). Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. The Journal of Clinical Investigation, 126(9), 3207–3218. https://doi.org/10.1172/jci86249
Kantarjian, H. M., Hughes, T. P., Larson, R. A., Kim, D. W., Issaragrisil, S., le Coutre, P., Etienne, G., Boquimpani, C., Pasquini, R., Clark, R. E., Dubruille, V., Flinn, I. W., Kyrcz-Krzemien, S., Medras, E., Zanichelli, M., Bendit, I., Cacciatore, S., Titorenko, K., Aimone, P., … Hochhaus, A. (2021). Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia, 35(2), 440–453. https://doi.org/10.1038/s41375-020-01111-2
Aichberger, K. J., Herndlhofer, S., Schernthaner, G. H., Schillinger, M., Mitterbauer-Hohendanner, G., Sillaber, C., & Valent, P. (2011). Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. American Journal of Hematology, 86(7), 533–539. https://doi.org/10.1002/ajh.22037
Hadzijusufovic, E., Albrecht-Schgoer, K., Huber, K., Hoermann, G., Grebien, F., Eisenwort, G., Schgoer, W., Herndlhofer, S., Kaun, C., Theurl, M., Sperr, W. R., Rix, U., Sadovnik, I., Jilma, B., Schernthaner, G. H., Wojta, J., Wolf, D., Superti-Furga, G., Kirchmair, R., & Valent, P. (2017). Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia, 31(11), 2388–2397. https://doi.org/10.1038/leu.2017.245
Kantarjian, H. M., Cortes, J. E., Kim, D. W., Khoury, H. J., Brümmendorf, T. H., Porkka, K., Martinelli, G., Durrant, S., Leip, E., Kelly, V., Turnbull, K., Besson, N., & Gambacorti-Passerini, C. (2014). Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood, 123(9), 1309–1318. https://doi.org/10.1182/blood-2013-07-513937
Hasinoff, B. B., & Patel, D. (2020). Mechanisms of the cardiac myocyte-damaging effects of dasatinib. Cardiovascular Toxicology, 20(4), 380–389. https://doi.org/10.1007/s12012-020-09565-7
Lekes, D., Szadvari, I., Krizanova, O., Lopusna, K., Rezuchova, I., Novakova, M., Novakova, Z., Parak, T., & Babula, P. (2016). Nilotinib induces ER stress and cell death in H9c2 cells. Physiological Research, 65(Suppl 4), 505–514. https://doi.org/10.33549/physiolres.933504
Chan, O., Talati, C., Isenalumhe, L., Shams, S., Nodzon, L., Fradley, M., Sweet, K., & Pinilla-Ibarz, J. (2020). Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Advances, 4(3), 530–538. https://doi.org/10.1182/bloodadvances.2019000268
Talbert, D. R., Doherty, K. R., Trusk, P. B., Moran, D. M., Shell, S. A., & Bacus, S. (2015). A multi-parameter in vitro screen in human stem cell-derived cardiomyocytes identifies ponatinib-induced structural and functional cardiac toxicity. Toxicological Sciences: An Official Journal of the Society of Toxicology, 143(1), 147–155. https://doi.org/10.1093/toxsci/kfu215
Paech, F., Mingard, C., Grünig, D., Abegg, V. F., Bouitbir, J., & Krähenbühl, S. (2018). Mechanisms of mitochondrial toxicity of the kinase inhibitors ponatinib, regorafenib and sorafenib in human hepatic HepG2 cells. Toxicology, 395, 34–44. https://doi.org/10.1016/j.tox.2018.01.005
Hughes, T. P., Mauro, M. J., Cortes, J. E., Minami, H., Rea, D., DeAngelo, D. J., Breccia, M., Goh, Y.-T., Talpaz, M., Hochhaus, A., le Coutre, P., Ottmann, O., Heinrich, M. C., Steegmann, J. L., Deininger, M. W. N., Janssen, J. J. W. M., Mahon, F.-X., Minami, Y., Yeung, D., … Kim, D.-W. (2019). Asciminib in chronic myeloid Leukemia after ABL kinase inhibitor failure. The New England Journal of Medicine, 381(24), 2315–2326. https://doi.org/10.1056/NEJMoa1902328
Rainbolt, T. K., Saunders, J. M., & Wiseman, R. L. (2014). Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends in endocrinology and metabolism: TEM, 25(10), 528–537. https://doi.org/10.1016/j.tem.2014.06.007
Gao, P., Yang, W., & Sun, L. (2020). Mitochondria-associated endoplasmic reticulum membranes (MAMs) and their prospective roles in kidney disease. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2020/3120539
Malhotra, J. D., & Kaufman, R. J. (2011). ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harbor Perspectives in Biology, 3(9), a004424. https://doi.org/10.1101/cshperspect.a004424
Turrens, J. F. (2003). Mitochondrial formation of reactive oxygen species. The Journal of Physiology, 552(Pt 2), 335–344. https://doi.org/10.1113/jphysiol.2003.049478
Rocca, C., De Francesco, E. M., Pasqua, T., Granieri, M. C., De Bartolo, A., Gallo Cantafio, M. E., Muoio, M. G., Gentile, M., Neri, A., Angelone, T., Viglietto, G., & Amodio, N. (2022). Mitochondrial determinants of anti-cancer drug-induced cardiotoxicity. Biomedicines. https://doi.org/10.3390/biomedicines10030520
Liu, Z. W., Zhu, H. T., Chen, K. L., Dong, X., Wei, J., Qiu, C., & Xue, J. H. (2013). Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovascular Diabetology, 12, 158. https://doi.org/10.1186/1475-2840-12-158
Ito, Y., Pandey, P., Mishra, N., Kumar, S., Narula, N., Kharbanda, S., Saxena, S., & Kufe, D. (2001). Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Molecular and Cellular Biology, 21(18), 6233–6242. https://doi.org/10.1128/mcb.21.18.6233-6242.2001
Pattacini, L., Mancini, M., Mazzacurati, L., Brusa, G., Benvenuti, M., Martinelli, G., Baccarani, M., & Santucci, M. A. (2004). Endoplasmic reticulum stress initiates apoptotic death induced by STI571 inhibition of p210 bcr-abl tyrosine kinase. Leukemia Research, 28(2), 191–202. https://doi.org/10.1016/s0145-2126(03)00218-2
Zhang, K., & Kaufman, R. J. (2004). Signaling the unfolded protein response from the endoplasmic reticulum. The Journal of Biological Chemistry, 279(25), 25935–25938. https://doi.org/10.1074/jbc.R400008200
Lozhkin, A., Vendrov, A. E., Ramos-Mondragón, R., Canugovi, C., Stevenson, M. D., Herron, T. J., Hummel, S. L., Figueroa, C. A., Bowles, D. E., Isom, L. L., Runge, M. S., & Madamanchi, N. R. (2022). Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics. Redox Biology, 57, 102474. https://doi.org/10.1016/j.redox.2022.102474
Dröse, S., & Brandt, U. (2012). Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Advances in Experimental Medicine and Biology. https://doi.org/10.1007/978-1-4614-3573-0_6
Liu, P., Xiang, J. Z., Zhao, L., Yang, L., Hu, B. R., & Fu, Q. (2008). Effect of beta2-adrenergic agonist clenbuterol on ischemia/reperfusion injury in isolated rat hearts and cardiomyocyte apoptosis induced by hydrogen peroxide. Acta Pharmacologica Sinica, 29(6), 661–669. https://doi.org/10.1111/j.1745-7254.2008.00794.x
Steinhorn, B., Sorrentino, A., Badole, S., Bogdanova, Y., Belousov, V., & Michel, T. (2018). Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nature Communications, 9(1), 4044. https://doi.org/10.1038/s41467-018-06533-2
Weatherald, J., Bondeelle, L., Chaumais, M. C., Guignabert, C., Savale, L., Jaïs, X., Sitbon, O., Rousselot, P., Humbert, M., Bergeron, A., & Montani, D. (2020). Pulmonary complications of Bcr-Abl tyrosine kinase inhibitors. The European Respiratory Journal. https://doi.org/10.1183/13993003.00279-2020
Damiano, S., Montagnaro, S., Puzio, M. V., Severino, L., Pagnini, U., Barbarino, M., Cesari, D., Giordano, A., Florio, S., & Ciarcia, R. (2018). Effects of antioxidants on apoptosis induced by dasatinib and nilotinib in K562 cells. Journal of Cellular Biochemistry, 119(6), 4845–4854. https://doi.org/10.1002/jcb.26686
Green, D. E., & Tzagoloff, A. (1966). The mitochondrial electron transfer chain. Archives of Biochemistry and Biophysics, 116(1), 293–304. https://doi.org/10.1016/0003-9861(66)90036-1
Chen, Q., Vazquez, E. J., Moghaddas, S., Hoppel, C. L., & Lesnefsky, E. J. (2003). Production of reactive oxygen species by mitochondria: Central role of complex III. The Journal of Biological Chemistry, 278(38), 36027–36031. https://doi.org/10.1074/jbc.M304854200
Morciano, G., Naumova, N., Koprowski, P., Valente, S., Sardão, V. A., Potes, Y., Rimessi, A., Wieckowski, M. R., & Oliveira, P. J. (2021). The mitochondrial permeability transition pore: An evolving concept critical for cell life and death. Biological Reviews of the Cambridge Philosophical Society, 96(6), 2489–2521. https://doi.org/10.1111/brv.12764
Kwong, J. Q., & Molkentin, J. D. (2015). Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metabolism, 21(2), 206–214. https://doi.org/10.1016/j.cmet.2014.12.001
Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S., & Wang, X. (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91(4), 479–489. https://doi.org/10.1016/s0092-8674(00)80434-1
Yang, Q., Zhang, C., Wei, H., Meng, Z., Li, G., Xu, Y., & Chen, Y. (2017). Caspase-independent pathway is related to nilotinib cytotoxicity in cultured cardiomyocytes. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 42(6), 2182–2193. https://doi.org/10.1159/000479993
Davis, R. J. (2000). Signal transduction by the JNK group of MAP kinases. Cell, 103(2), 239–252. https://doi.org/10.1016/s0092-8674(00)00116-1
Urano, F., Wang, X., Bertolotti, A., Zhang, Y., Chung, P., Harding, H. P., & Ron, D. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science (New York, N.Y.), 287(5453), 664–666. https://doi.org/10.1126/science.287.5453.664
Chambers, J. W., & LoGrasso, P. V. (2011). Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. The Journal of Biological Chemistry, 286(18), 16052–16062. https://doi.org/10.1074/jbc.M111.223602
Yang, Q., Wen, L., Meng, Z., & Chen, Y. (2018). Blockage of endoplasmic reticulum stress attenuates nilotinib-induced cardiotoxicity by inhibition of the Akt-GSK3β-Nox4 signaling. European Journal of Pharmacology, 822, 85–94. https://doi.org/10.1016/j.ejphar.2018.01.011
Chambers, T. P., Santiesteban, L., Gomez, D., & Chambers, J. W. (2017). Sab mediates mitochondrial dysfunction involved in imatinib mesylate-induced cardiotoxicity. Toxicology, 382, 24–35. https://doi.org/10.1016/j.tox.2017.03.006
Chaar, M., Kamta, J., & Ait-Oudhia, S. (2018). Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. OncoTargets and Therapy, 11, 6227–6237. https://doi.org/10.2147/ott.S170138
Freebern, W. J., Fang, H. S., Slade, M. D., Wells, S., Canale, J., Megill, J., Grubor, B., Shi, H., Fletcher, A., Lombardo, L., Levesque, P., Lee, F. Y., & Sasseville, V. G. (2007). In vitro cardiotoxicity potential comparative assessments of chronic myelogenous leukemia tyrosine kinase inhibitor therapies: Dasatinib imatinib and nilotinib. Blood, 110(11), 4582. https://doi.org/10.1182/blood.V110.11.4582.4582
Wolf, A., Couttet, P., Dong, M., Grenet, O., Heron, M., Junker, U., Laengle, U., Ledieu, D., Marrer, E., Nussher, A., Persohn, E., Pognan, F., Rivière, G. J., Roth, D. R., Trendelenburg, C., Tsao, J., & Roman, D. (2010). Imatinib does not induce cardiotoxicity at clinically relevant concentrations in preclinical studies. Leukemia Research, 34(9), 1180–1188. https://doi.org/10.1016/j.leukres.2010.01.004
Hasinoff, B. B., Patel, D., & Wu, X. (2017). The myocyte-damaging effects of the BCR-ABL1-targeted tyrosine kinase inhibitors increase with potency and decrease with specificity. Cardiovascular Toxicology, 17(3), 297–306. https://doi.org/10.1007/s12012-016-9386-7
Kobayashi, S., Lackey, T., Huang, Y., Bisping, E., Pu, W. T., Boxer, L. M., & Liang, Q. (2006). Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 20(6), 800–802. https://doi.org/10.1096/fj.05-5426fje
Sarszegi, Z., Bognar, E., Gaszner, B., Kónyi, A., Gallyas, F., Jr., Sumegi, B., & Berente, Z. (2012). BGP-15, a PARP-inhibitor, prevents imatinib-induced cardiotoxicity by activating Akt and suppressing JNK and p38 MAP kinases. Molecular and Cellular Biochemistry, 365(1–2), 129–137. https://doi.org/10.1007/s11010-012-1252-8
Xu, Z., Jin, Y., Yan, H., Gao, Z., Xu, B., Yang, B., He, Q., Shi, Q., & Luo, P. (2018). High-mobility group box 1 protein-mediated necroptosis contributes to dasatinib-induced cardiotoxicity. Toxicology Letters, 296, 39–47. https://doi.org/10.1016/j.toxlet.2018.08.003
Alsaad, A. M. S. (2018). Dasatinib induces gene expression of CYP1A1, CYP1B1, and cardiac hypertrophy markers (BNP, β-MHC) in rat cardiomyocyte H9c2 cells. Toxicology Mechanisms and Methods, 28(9), 678–684. https://doi.org/10.1080/15376516.2018.1497746
Madonna, R., Pieragostino, D., Cufaro, M. C., Del Boccio, P., Pucci, A., Mattii, L., Doria, V., Cadeddu Dessalvi, C., Zucchi, R., Mercuro, G., & De Caterina, R. (2022). Sex-related differential susceptibility to ponatinib cardiotoxicity and differential modulation of the Notch1 signalling pathway in a murine model. Journal of Cellular and Molecular Medicine, 26(5), 1380–1391. https://doi.org/10.1111/jcmm.17008
Singh, A. P., Glennon, M. S., Umbarkar, P., Gupte, M., Galindo, C. L., Zhang, Q., Force, T., Becker, J. R., & Lal, H. (2019). Ponatinib-induced cardiotoxicity: Delineating the signalling mechanisms and potential rescue strategies. Cardiovascular Research, 115(5), 966–977. https://doi.org/10.1093/cvr/cvz006
Sharma, A., Burridge, P. W., McKeithan, W. L., Serrano, R., Shukla, P., Sayed, N., Churko, J. M., Kitani, T., Wu, H. D., Holmstrom, A., Matsa, E., Zhang, Y., Kumar, A., Fan, A. C., del Alamo, J. C., Wu, S. M., Moslehi, J. J., Mercola, M., & Wu, J. C. (2017). High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Science Translational Medicine. https://doi.org/10.1126/scitranslmed.aaf2584
Latifi, Y., Moccetti, F., Wu, M., Xie, A., Packwood, W., Qi, Y., Ozawa, K., Shentu, W. H., Brown, E., Shirai, T., McCarty, O. J., Ruggeri, Z., Moslehi, J., Chen, J. M., Druker, B. J., Lopez, J. A., & Lindner, J. R. (2019). Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood, 133(14), 1597–1606. https://doi.org/10.1182/blood-2018-10-881557
Hadzijusufovic, E., Kirchmair, R., Theurl, M., Gamperl, S., Lener, D., Gutmann, C., Stanzl, U., Kirsch, A., Frank, S., & Valent, P. (2016). Ponatinib exerts multiple effects on vascular endothelial cells: Possible mechanisms and explanations for the adverse vascular events seen in CML patients treated with ponatinib. Blood, 128(22), 1883. https://doi.org/10.1182/blood.V128.22.1883.1883
Durand, M. J., Hader, S. N., Derayunan, A., Zinkevich, N., McIntosh, J. J., & Beyer, A. M. (2020). BCR-ABL tyrosine kinase inhibitors promote pathological changes in dilator phenotype in the human microvasculature. Microcirculation. https://doi.org/10.1111/micc.12625
Zhou, S. B., Wang, J., & Liu, H. (2016). Lead compound optimization strategy(5) – reducing the hERG cardiac toxicity in drug development. Yao xue xue bao = Acta pharmaceutica Sinica, 51(10), 1530–1539.
Hasinoff, B. B., & Patel, D. (2010). Mechanisms of myocyte cytotoxicity induced by the multikinase inhibitor sorafenib. Cardiovascular Toxicology, 10(1), 1–8. https://doi.org/10.1007/s12012-009-9056-0
Aghel, N., Delgado, D. H., & Lipton, J. H. (2017). Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: Preventive strategies and cardiovascular surveillance. Vascular Health and Risk Management, 13, 293–303. https://doi.org/10.2147/vhrm.S108874
Özgür Yurttaş, N., & Eşkazan, A. E. (2018). Dasatinib-induced pulmonary arterial hypertension. British Journal of Clinical Pharmacology, 84(5), 835–845. https://doi.org/10.1111/bcp.13508
Oikonomou, E. K., Kokkinidis, D. G., Kampaktsis, P. N., Amir, E. A., Marwick, T. H., Gupta, D., & Thavendiranathan, P. (2019). Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: A systematic review and meta-analysis. JAMA Cardiology, 4(10), 1007–1018. https://doi.org/10.1001/jamacardio.2019.2952
Novo, G., Di Lisi, D., Bronte, E., Macaione, F., Accurso, V., Badalamenti, G., Rinaldi, G., Siragusa, S., Novo, S., & Russo, A. (2020). Cardiovascular toxicity in cancer patients treated with tyrosine kinase inhibitors: A real-world single-center experience. Oncology, 98(7), 445–451. https://doi.org/10.1159/000505486
Thavendiranathan, P., Negishi, T., Somerset, E., Negishi, K., Penicka, M., Lemieux, J., Aakhus, S., Miyazaki, S., Shirazi, M., Galderisi, M., & Marwick, T. H. (2021). Strain-guided management of potentially cardiotoxic cancer therapy. Journal of the American College of Cardiology, 77(4), 392–401. https://doi.org/10.1016/j.jacc.2020.11.020
Breccia, M., Pregno, P., Spallarossa, P., Arboscello, E., Ciceri, F., Giorgi, M., Grossi, A., Mallardo, M., Nodari, S., Ottolini, S., Sala, C., Tortorella, G., Rosti, G., Pane, F., Minotti, G., & Baccarani, M. (2017). Identification, prevention and management of cardiovascular risk in chronic myeloid leukaemia patients candidate to ponatinib: An expert opinion. Annals of Hematology, 96(4), 549–558. https://doi.org/10.1007/s00277-016-2820-x
Caocci, G., Mulas, O., Abruzzese, E., Luciano, L., Iurlo, A., Attolico, I., Castagnetti, F., Galimberti, S., Sgherza, N., Bonifacio, M., Annunziata, M., Gozzini, A., Orlandi, E. M., Stagno, F., Binotto, G., Pregno, P., Fozza, C., Trawinska, M. M., De Gregorio, F., … Breccia, M. (2019). Arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life practice are predicted by the Systematic Coronary Risk Evaluation (SCORE) chart. Hematological Oncology, 37(3), 296–302. https://doi.org/10.1002/hon.2606
Caocci, G., Mulas, O., Capodanno, I., Bonifacio, M., Annunziata, M., Galimberti, S., Luciano, L., Tiribelli, M., Martino, B., Castagnetti, F., Binotto, G., Pregno, P., Stagno, F., Abruzzese, E., Bocchia, M., Gozzini, A., Albano, F., Fozza, C., Luzi, D., … La Nasa, G. (2021). Low-density lipoprotein (LDL) levels and risk of arterial occlusive events in chronic myeloid leukemia patients treated with nilotinib. Annals of Hematology, 100(8), 2005–2014. https://doi.org/10.1007/s00277-020-04392-w
Di Lisi, D., Madaudo, C., Alagna, G., Santoro, M., Rossetto, L., Siragusa, S., & Novo, G. (2022). The new HFA/ICOS risk assessment tool to identify patients with chronic myeloid leukaemia at high risk of cardiotoxicity. ESC Heart Failure, 9(3), 1914–1919. https://doi.org/10.1002/ehf2.13897
Fernández, A., Sanguino, A., Peng, Z., Ozturk, E., Chen, J., Crespo, A., Wulf, S., Shavrin, A., Qin, C., Ma, J., Trent, J., Lin, Y., Han, H. D., Mangala, L. S., Bankson, J. A., Gelovani, J., Samarel, A., Bornmann, W., Sood, A. K., & Lopez-Berestein, G. (2007). An anticancer C-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. The Journal of Clinical Investigation, 117(12), 4044–4054. https://doi.org/10.1172/jci32373
Demetri, G. D. (2007). Structural reengineering of imatinib to decrease cardiac risk in cancer therapy. Journal of Clinical Investigation, 117(12), 3650–3653. https://doi.org/10.1172/jci34252
Hnatiuk, A. P., Bruyneel, A. A. N., Tailor, D., Pandrala, M., Dheeraj, A., Li, W., Serrano, R., Feyen, D. A. M., Vu, M. M., Amatya, P., Gupta, S., Nakauchi, Y., Morgado, I., Wiebking, V., Liao, R., Porteus, M. H., Majeti, R., Malhotra, S. V., & Mercola, M. (2022). Reengineering Ponatinib to Minimize Cardiovascular Toxicity. Cancer Research, 82(15), 2777–2791. https://doi.org/10.1158/0008-5472.CAN-21-3652
Guo, Y., Wang, X., Jia, P., You, Y., Cheng, Y., Deng, H., Luo, S., & Huang, B. (2020). Ketogenic diet aggravates hypertension via NF-κB-mediated endothelial dysfunction in spontaneously hypertensive rats. Life Sci, 258, 118124. https://doi.org/10.1016/j.lfs.2020.118124
Qu, C., Keijer, J., Adjobo-Hermans, M. J. W., van de Wal, M., Schirris, T., van Karnebeek, C., Pan, Y., & Koopman, W. J. H. (2021). The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. The International Journal of Biochemistry & Cell Biology, 138, 106050. https://doi.org/10.1016/j.biocel.2021.106050
Balietti, M., Fattoretti, P., Giorgetti, B., Casoli, T., Di Stefano, G., Solazzi, M., Platano, D., Aicardi, G., & Bertoni-Freddari, C. (2009). A ketogenic diet increases succinic dehydrogenase activity in aging cardiomyocytes. Annals of the New York Academy of Sciences, 1171, 377–384. https://doi.org/10.1111/j.1749-6632.2009.04704.x
Yu, Y., Wang, F., Wang, J., Zhang, D., & Zhao, X. (2020). Ketogenic diet attenuates aging-associated myocardial remodeling and dysfunction in mice. Experimental gerontology, 140, 111058. https://doi.org/10.1016/j.exger.2020.111058
Guo, Y., Zhang, C., Shang, F.-F., Luo, M., You, Y., Zhai, Q., Xia, Y., & Suxin, L. (2020). Ketogenic Diet ameliorates cardiac dysfunction via balancing mitochondrial dynamics and Inhibiting apoptosis apoptosis in type 2 diabetic. Aging Dis, 11(2), 229–240. https://doi.org/10.14336/AD.2019.0510
Lopaschuk, G. D., Karwi, Q. G., Ho, K. L., Pherwani, S., & Ketema, E. B. (2020). Ketone metabolism in the failing heart. Biochimica et Biophysica acta Molecular and cell biology of lipids, 1865(12), 158813. https://doi.org/10.1016/j.bbalip.2020.158813
Al-Zaid, N. S., Dashti, H. M., Mathew, T. C., & Juggi, J. S. (2007). Low carbohydrate ketogenic diet enhances cardiac tolerance to global ischaemia. Acta Cardiologica, 62(4), 381–389.
Hasan-Olive, M. M., Lauritzen, K. H., Ali, M., Rasmussen, L. J., Storm-Mathisen, J., & Bergersen, L. H. (2019). A ketogenic diet improves mitochondrial biogenesis and bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochemical Research, 44(1), 22–37. https://doi.org/10.1007/s11064-018-2588-6
Ji, L., He, Q., Liu, Y., Deng, Y., Xie, M., Luo, K., Cai, X., Zuo, Y., Wu, W., Li, Q., Zhou, R., & Li, T. (2022). Ketone Body -hydroxybutyrate prevents myocardial oxidative stress in septic cardiomyopathy. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2022/2513837
Ascensão, A., Ferreira, R., & Magalhães, J. (2007). Exercise-induced cardioprotection–biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. International Journal of Cardiology, 117(1), 16–30. https://doi.org/10.1016/j.ijcard.2006.04.076
Boulghobra, D., Coste, F., Geny, B., & Reboul, C. (2020). Exercise training protects the heart against ischemia-reperfusion injury: A central role for mitochondria? Free Radical Biology & Medicine, 152, 395–410. https://doi.org/10.1016/j.freeradbiomed.2020.04.005
Boulghobra, D., Dubois, M., Alpha-Bazin, B., Coste, F., Olmos, M., Gayrard, S., Bornard, I., Meyer, G., Gaillard, J. C., Armengaud, J., & Reboul, C. (2021). Increased protein S-nitrosylation in mitochondria: A key mechanism of exercise-induced cardioprotection. Basic research in cardiology, 116(1), 66. https://doi.org/10.1007/s00395-021-00906-3
Lee, Y., Min, K., Talbert, E. E., Kavazis, A. N., Smuder, A. J., Willis, W. T., & Powers, S. K. (2012). Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Medicine and Science in Sports and Exercise, 44(3), 397–405. https://doi.org/10.1249/MSS.0b013e318231c037
Kavazis, A. N., McClung, J. M., Hood, D. A., & Powers, S. K. (2008). Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. American Journal of Physiology Heart and Circulatory Physiology, 294(2), 928–935. https://doi.org/10.1152/ajpheart.01231.2007
Chang, X., Liu, J., Wang, Y., Guan, X., & Liu, R. (2023). Mitochondrial disorder and treatment of ischemic cardiomyopathy: Potential and advantages of Chinese herbal medicine. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 159, 114171. https://doi.org/10.1016/j.biopha.2022.114171
Jiang, L., Yin, X., Chen, Y. H., Chen, Y., Jiang, W., Zheng, H., Huang, F. Q., Liu, B., Zhou, W., Qi, L. W., & Li, J. (2021). Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics, 11(4), 1703–1720. https://doi.org/10.7150/thno.43895
Dong, G., Chen, T., Ren, X., Zhang, Z., Huang, W., Liu, L., Luo, P., & Zhou, H. (2016). Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion. https://doi.org/10.1016/j.mito.2015.11.003
Quinsay, M. N., Thomas, R. L., Lee, Y., & Gustafsson, A. B. (2010). Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy, 6(7), 855–862. https://doi.org/10.4161/auto.6.7.13005
Liu, C. S., Chang, J. C., Kuo, S. J., Liu, K. H., Lin, T. T., Cheng, W. L., & Chuang, S. F. (2014). Delivering healthy mitochondria for the therapy of mitochondrial diseases and beyond. The International Journal of Biochemistry & Cell Biology, 53, 141–146. https://doi.org/10.1016/j.biocel.2014.05.009
McCully, J. D., Cowan, D. B., Pacak, C. A., Toumpoulis, I. K., Dayalan, H., & Levitsky, S. (2009). Injection of isolated mitochondria during early reperfusion for cardioprotection. American Journal Of Physiology Heart and Circulatory Physiology, 296(1), H94-h105. https://doi.org/10.1152/ajpheart.00567.2008
Shin, B., Cowan, D. B., Emani, S. M., Del Nido, P. J., & McCully, J. D. (2017). Mitochondrial Transplantation in Myocardial Ischemia and Reperfusion Injury. Advances in Experimental Medicine and Biology. https://doi.org/10.1007/978-3-319-55330-6_31
Ross, D. M., & Hughes, T. P. (2020). Treatment-free remission in patients with chronic myeloid leukaemia. Nature reviews Clinical oncology, 17(8), 493–503. https://doi.org/10.1038/s41571-020-0367-1
Sato, E., Iriyama, N., Tokuhira, M., Takaku, T., Ishikawa, M., Nakazato, T., Sugimoto, K. J., Fujita, H., Kimura, Y., Fujioka, I., Asou, N., Komatsu, N., Kizaki, M., Hatta, Y., & Kawaguchi, T. (2020). The EUTOS long-term survival score predicts disease-specific mortality and molecular responses among patients with chronic myeloid leukemia in a practice-based cohort. Cancer Medicine, 9(23), 8931–8939. https://doi.org/10.1002/cam4.3516
Hochhaus, A., Baccarani, M., Silver, R. T., Schiffer, C., Apperley, J. F., Cervantes, F., Clark, R. E., Cortes, J. E., Deininger, M. W., Guilhot, F., Hjorth-Hansen, H., Hughes, T. P., Janssen, J., Kantarjian, H. M., Kim, D. W., Larson, R. A., Lipton, J. H., Mahon, F. X., Mayer, J., … Hehlmann, R. (2020). European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia, 34(4), 966–984. https://doi.org/10.1038/s41375-020-0776-2
Vaidya, T., Kamta, J., Chaar, M., Ande, A., & Ait-Oudhia, S. (2018). Systems pharmacological analysis of mitochondrial cardiotoxicity induced by selected tyrosine kinase inhibitors. Journal of Pharmacokinetics and Pharmacodynamics, 45(3), 401–418. https://doi.org/10.1007/s10928-018-9578-9
Henkin, R. I. (2019). Clinical and therapeutic implications of cancer stem cells. New England Journal of Medicine, 381(10), 19. https://doi.org/10.1056/NEJMc1908886
Schoepfer, J., Jahnke, W., Berellini, G., Buonamici, S., Cotesta, S., Cowan-Jacob, S. W., Dodd, S., Drueckes, P., Fabbro, D., Gabriel, T., Groell, J.-M., Grotzfeld, R. M., Hassan, A. Q., Henry, C., Iyer, V., Jones, D., Lombardo, F., Loo, A., Manley, P. W., … Furet, P. (2018). Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. Journal of Medicinal Chemistry, 61(18), 8120–8135. https://doi.org/10.1021/acs.jmedchem.8b01040
Réa, D., Mauro, M. J., Boquimpani, C., Minami, Y., Lomaia, E., Voloshin, S., Turkina, A., Kim, D. W., Apperley, J. F., Abdo, A., Fogliatto, L. M., Kim, D. D. H., le Coutre, P., Saussele, S., Annunziata, M., Hughes, T. P., Chaudhri, N., Sasaki, K., Chee, L., … Hochhaus, A. (2021). A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, 138(21), 2031–2041. https://doi.org/10.1182/blood.2020009984
Kantarjian, H., Paul, S., Thakkar, J., & Jabbour, E. (2022). The influence of drug prices, new availability of inexpensive generic imatinib, new approvals, and post-marketing research on the treatment of chronic myeloid leukaemia in the USA. The Lancet Haematology, 9(11), 854–861. https://doi.org/10.1016/s2352-3026(22)00246-0
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparation of the the first draft of the manuscript. All authors also approved the final format of the article.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research Involving Human and Animal Rights participants
Research Involving Human and Animal Rights. This article does not contain human or animal studies performed by any of the authors.
Informed Consent
Not Applicable.
Additional information
Handling Editor: Daniel Conklin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, S., Qin, J., Liao, W. et al. Mitochondrial Dysfunction in Cardiotoxicity Induced by BCR-ABL1 Tyrosine Kinase Inhibitors -Underlying Mechanisms, Detection, Potential Therapies. Cardiovasc Toxicol 23, 233–254 (2023). https://doi.org/10.1007/s12012-023-09800-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-023-09800-x