Abstract
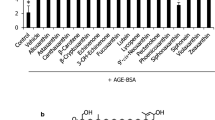
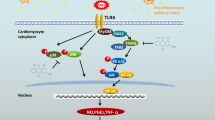
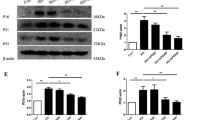
Accumulating evidences have demonstrated that increased production of advanced glycation end products (AGEs) contributes to etiology of cardiac complications in diabetes. However, the underlying mechanism of AGE-induced effects is not well understood. Recent studies evince the beneficial role of phytochemicals in reducing the risk of cardiovascular morbidity and mortality in patients with cardiovascular diseases and diabetes mellitus. Hence, in the present study, the cardio protective role of gallic acid (GA) against in vitro synthesized AGE in H9C2 (2-1) cells was elucidated. H9C2 (2-1) cells exposed to AGE (100 μg/ml) with/without GA pre-treatment (10 μM) and the release of reactive oxygen species (ROS), expression of oxidative stress markers, matrix proteins, and cytokines were analyzed. Cells exposed to AGE demonstrate a significant increase in ROS release with augmented expression (P < 0.01) of receptor for AGE (RAGE) and NOX-p47 phox (P < 0.001) proteins compared to untreated control cells. Moreover, an increased expression of matrix proteins and cytokines such as TNF-α (P < 0.01), TGF-β (P < 0.001), and iNOS (P < 0.001) was also found in AGE-treated cells, whereas, cells pre-treated with N-acetyl cysteine or RAGE neutralizing antibody notably (P < 0.01) impede the ROS release. Further, cells pre-treated with GA significantly attenuated the expression of NOX, RAGE, and other cytokines. In addition, the abnormal expressions of matrix proteins were also decreased especially in GA-treated cells. Thus, the results of the present study demonstrated the deleterious effect of AGEs that directly induce oxidative stress and matrix derangement and, on the other way, the “pleiotropic” activity of GA in reducing the risk of AGE-mediated cellular complications.







Similar content being viewed by others
References
Thornalley, J. P. (1996). Advanced glycation and the development of diabetic complications: Unifying the involvement of glucose, methylglyoxal and oxidative stress. Endocrinology and Metabolism-London, 3, 149–166.
Singh, R., Barden, A., Mori, T., & Beilin, L. (2001). Advanced glycation end-products: A review. Diabetologia, 44, 129–146.
Aronson, D. (2003). Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. Journal of Hypertension, 21, 3–12.
Thallas-Bonke, V., Lindschau, C., Rizkalla, B., Bach, L. A., Boner, G., Meier, M., et al. (2004). Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase C-alpha-dependent pathway. Diabetes, 53, 2921–2930.
Corman, B., Duriez, M., Poitevin, P., Heudes, D., Bruneval, P., Tedgui, A., et al. (1998). Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America, 95, 1301–1306.
Hartog, J. W., Voors, A. A., Bakker, S. J., Smit, A. J., & van Veldhuisen, D. J. (2007). Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. European Journal of Heart Failure, 9, 1146–1155.
Huang, J. S., Guh, J. Y., Hung, W. C., Yang, M. L., Lai, Y. H., Chen, H. C., et al. (1999). Role of the Janus kinase (JAK)/signal transducters and activators of transcription (STAT) cascade in advanced glycation end-product-induced cellular mitogenesis in NRK-49F cells. The Biochemical Journal, 342(Pt 1), 231–238.
Lohwasser, C., Neureiter, D., Popov, Y., Bauer, M., & Schuppan, D. (2009). Role of the receptor for advanced glycation end products in hepatic fibrosis. World Journal of Gastroenterology, 15, 5789–5798.
Bierhaus, A., Chevion, S., Chevion, M., Hofmann, M., Quehenberger, P., Illmer, T., et al. (1997). Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes, 46, 1481–1490.
Schmidt, A. M., Mora, R., Cao, R., Yan, S. D., Brett, J., Ramakrishnan, R., et al. (1994). The endothelial cell binding site for advanced glycation end products consists of a complex: An integral membrane protein and a lactoferrin-like polypeptide. The Journal of Biological Chemistry, 269, 9882–9888.
Lander, H. M., Tauras, J. M., Ogiste, J. S., Hori, O., Moss, R. A., & Schmidt, A. M. (1997). Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. The Journal of Biological Chemistry, 272, 17810–17814.
Stern, D., Yan, S. D., Yan, S. F., & Schmidt, A. M. (2002). Receptor for advanced glycation endproducts: A multiligand receptor magnifying cell stress in diverse pathologic settings. Advanced Drug Delivery Reviews, 54, 1615–1625.
Jagan, S., Ramakrishnan, G., Anandakumar, P., Kamaraj, S., & Devaki, T. (2008). Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Molecular and Cellular Biochemistry, 319, 51–59.
Pal, C., Bindu, S., Dey, S., Alam, A., Goyal, M., Iqbal, M. S., et al. (2010). Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radical Biology and Medicine, 49, 258–267.
Priscilla, D. H., & Prince, P. S. (2009). Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chemico-Biological Interactions, 179, 118–124.
Umadevi, S., Gopi, V., Simna, S. P., Parthasarathy, A., Yousuf, S. M., & Elangovan, V. (2012). Studies on the cardio protective role of gallic acid against AGE-induced cell proliferation and oxidative stress in H9C2 (2-1) cells. Cardiovascular Toxicology, 12, 304–311.
Andreea, S. I., Marieta, C., & Anca, D. (2008). AGEs and glucose levels modulate type I and III procollagen mRNA synthesis in dermal fibroblasts cells culture. Experimental Diabetes Research, 2008, 473603.
Stathopoulou, K., Beis, I., & Gaitanaki, C. (2008). MAPK signaling pathways are needed for survival of H9c2 cardiac myoblasts under extracellular alkalosis. American Journal of Physiology, Heart and Circulatory Physiology, 295, H1319–H1329.
Lee, H. B., Yu, M. R., Yang, Y., Jiang, Z., & Ha, H. (2003). Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. Journal of the American Society of Nephrology, 14, S241–S245.
Pamplona, R. (2008). Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochimica et Biophysica Acta, 1777, 1249–1262.
Ambati, J., Ambati, B. K., Yoo, S. H., Ianchulev, S., & Adamis, A. P. (2003). Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Survey of Ophthalmology, 48, 257–293.
Lee, S. J., & Lee, K. W. (2007). Protective effect of (-)-epigallocatechin gallate against advanced glycation endproducts-induced injury in neuronal cells. Biological and Pharmaceutical Bulletin, 30, 1369–1373.
Nakagawa, T., Yokozawa, T., Terasawa, K., Shu, S., & Juneja, L. R. (2002). Protective activity of green tea against free radical- and glucose-mediated protein damage. Journal of Agricultural and Food Chemistry, 50, 2418–2422.
Peters, C. A., Freeman, M. R., Fernandez, C. A., Shepard, J., Wiederschain, D. G., & Moses, M. A. (1997). Dysregulated proteolytic balance as the basis of excess extracellular matrix in fibrotic disease. The American Journal of Physiology, 272, R1960–R1965.
Steed, M. M., Tyagi, N., Sen, U., Schuschke, D. A., Joshua, I. G., & Tyagi, S. C. (2010). Functional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS-/-, and iNOS-/- mice. American journal of physiology, Lung Cellular and Molecular Physiology, 299, L301–L311.
Camelliti, P., Borg, T. K., & Kohl, P. (2005). Structural and functional characterisation of cardiac fibroblasts. Cardiovascular Research, 65, 40–51.
Death, A. K., Fisher, E. J., McGrath, K. C., & Yue, D. K. (2003). High glucose alters matrix metalloproteinase expression in two key vascular cells: Potential impact on atherosclerosis in diabetes. Atherosclerosis, 168, 263–269.
Tarallo, S., Beltramo, E., Berrone, E., Dentelli, P., & Porta, M. (2010). Effects of high glucose and thiamine on the balance between matrix metalloproteinases and their tissue inhibitors in vascular cells. Acta Diabetologica, 47, 105–111.
Thallas-Bonke, V., Thorpe, S. R., Coughlan, M. T., Fukami, K., Yap, F. Y., Sourris, K. C., et al. (2008). Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes, 57, 460–469.
Li, J. H., Huang, X. R., Zhu, H. J., Johnson, R., & Lan, H. Y. (2003). Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney International, 63, 2010–2019.
Ihn, H. (2002). Pathogenesis of fibrosis: Role of TGF-beta and CTGF. Current Opinion in Rheumatology, 14, 681–685.
Khan, R., & Sheppard, R. (2006). Fibrosis in heart disease: Understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology, 118, 10–24.
Lassegue, B., & Clempus, R. E. (2003). Vascular NAD(P)H oxidases: Specific features, expression, and regulation. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology, 285, R277–R297.
Sangle, G. V., Zhao, R., Mizuno, T. M., & Shen, G. X. (2010). Involvement of RAGE, NADPH oxidase, and Ras/Raf-1 pathway in glycated LDL-induced expression of heat shock factor-1 and plasminogen activator inhibitor-1 in vascular endothelial cells. Endocrinology, 151, 4455–4466.
Bedard, K., & Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiological Reviews, 87, 245–313.
San Martin, A., Foncea, R., Laurindo, F. R., Ebensperger, R., Griendling, K. K., & Leighton, F. (2007). Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radical Biology and Medicine, 42, 1671–1679.
Zafari, A. M., Ushio-Fukai, M., Akers, M., Yin, Q., Shah, A., Harrison, D. G., et al. (1998). Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension, 32, 488–495.
Pacher, P., Beckman, J. S., & Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiological Reviews, 87, 315–424.
Ricciardolo, F. L., Sterk, P. J., Gaston, B., & Folkerts, G. (2004). Nitric oxide in health and disease of the respiratory system. Physiological Reviews, 84, 731–765.
Gao, X., Belmadani, S., Picchi, A., Xu, X., Potter, B. J., Tewari-Singh, N., et al. (2007). Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation, 115, 245–254.
Csiszar, A., & Ungvari, Z. (2008). Endothelial dysfunction and vascular inflammation in type 2 diabetes: Interaction of AGE/RAGE and TNF-alpha signaling. American Journal of Physiology, Heart and Circulatory Physiology, 295, H475–H476.
Mamputu, J. C., & Renier, G. (2004). Advanced glycation end-products increase monocyte adhesion to retinal endothelial cells through vascular endothelial growth factor-induced ICAM-1 expression: Inhibitory effect of antioxidants. Journal of Leukocyte Biology, 75, 1062–1069.
Brownlee, M. (1995). Advanced protein glycosylation in diabetes and aging. Annual Review of Medicine, 46, 223–234.
Brownlee, M., Cerami, A., & Vlassara, H. (1988). Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. The New England journal of medicine, 318, 1315–1321.
Park, S., Yoon, S. J., Tae, H. J., & Shim, C. Y. (2011). RAGE and cardiovascular disease. Frontiers in Bioscience: A Journal and Virtual Library, 16, 486–497.
Forbes, J. M., Coughlan, M. T., & Cooper, M. E. (2008). Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes, 57, 1446–1454.
Brasier, A. R. (2010). The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovascular Research, 86, 211–218.
Frier, B. C., Noble, E. G., & Locke, M. (2008). Diabetes-induced atrophy is associated with a muscle-specific alteration in NF-kappaB activation and expression. Cell Stress and Chaperones, 13, 287–296.
Van der Heiden, K., Cuhlmann, S., le Luong, A., Zakkar, M., & Evans, P. C. (2010). Role of nuclear factor kappaB in cardiovascular health and disease. Clinical Science, 118, 593–605. (London, England: 1979).
Evans, J. L., Goldfine, I. D., Maddux, B. A., & Grodsky, G. M. (2002). Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocrine Reviews, 23, 599–622.
Zhang, W., & Liu, H. T. (2002). MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research, 12, 9–18.
Roux, P. P., & Blenis, J. (2004). ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiology and Molecular Biology Reviews, 68, 320–344.
Oeckinghaus, A., Hayden, M. S., & Ghosh, S. (2011). Crosstalk in NF-kappaB signaling pathways. Nature Immunology, 12, 695–708.
Li, S. Y., Sigmon, V. K., Babcock, S. A., & Ren, J. (2007). Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sciences, 80, 1051–1056.
Acknowledgments
This work was supported by JNMF and CSIR, New Delhi, India.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umadevi, S., Gopi, V. & Vellaichamy, E. Inhibitory Effect of Gallic Acid on Advanced Glycation End Products Induced Up-Regulation of Inflammatory Cytokines and Matrix Proteins in H9C2 (2-1) Cells. Cardiovasc Toxicol 13, 396–405 (2013). https://doi.org/10.1007/s12012-013-9222-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9222-2