Abstract
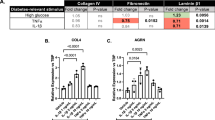
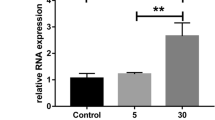
Pericyte survival in diabetic retinopathy depends also on interactions with extracellular matrix (ECM) proteins, which are degraded by matrix metalloproteinases (MMP). Elevated glucose influences ECM turnover, through expression of MMP and their tissue inhibitors, TIMP. We reported on reduced pericyte adhesion to high glucose-conditioned ECM and correction by thiamine. We aimed at verifying the effects of thiamine and benfotiamine on MMP-2, MMP-9 and TIMP expression and activity in human vascular cells with high glucose. In HRP, MMP-2 activity, though not expression, increased with high glucose and decreased with thiamine and benfotiamine; TIMP-1 expression increased with high glucose plus thiamine and benfotiamine; MMP-9 was not expressed. In EC, MMP-9 and MMP-2 expression and activity increased with high glucose, but thiamine and benfotiamine had no effects; TIMP-1 expression was unchanged. Neither glucose nor thiamine modified TIMP-2 and TIMP-3 expression. TIMP-1 concentrations did not change in either HRP or EC. High glucose imbalances MMP/TIMP regulation, leading to increased ECM turnover. Thiamine and benfotiamine correct the increase in MMP-2 activity due to high glucose in HRP, while increasing TIMP-1.


Similar content being viewed by others
References
Williams FM, Dosso AA, Kohner EM, Porta M (1993) Pericyte mitogenic activity is reduced in the blood of type 1 diabetic patients with and without retinopathy. Acta Diabetol 30:123–127
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523
Hammes HP (2005) Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 37:39–43
Yang R, Liu H, Williams I, Chaqour B (2007) Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann NY Acad Sci 110:196–201
Borden P, Heller RA (1997) Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr 7:159–178
Nagase H (1997) Activation mechanisms of matrix metalloproteinases. Biol Chem 378:151–160
Fisher E, McLennan SV, Tada H, Heffernan S, Yue DK, Turtle JR (1991) Interaction of ascorbic acid and glucose on production of collagen and proteoglycan by fibroblasts. Diabetes 40:371–376
Pugliese G, Pricci F, Pugliese F, Mene P, Lenti L, Andreani D, Galli G, Casini A, Bianchi S, Rotella CM (1994) Mechanisms of glucose-enhanced extracellular matrix accumulation in rat glomerular mesangial cells. Diabetes 43:478–490
Death AK, Fisher EJ, McGrath KCY, Yue DK (2003) High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis 168:263–269
Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD (2005) Matrix metalloproteinases and diabetic vascular complications. Angiology 56:173–189
Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB (2006) Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res 99:140–148
Hao F, Yu JD (2003) High glucose enhances expression of matrix metalloproteinase-2 in smooth muscle cells. Acta Pharmacol Sin 24:534–538
Nishikawa T, Edelstein D, Brownlee M (2000) The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl (77):S26–S30
La Selva M, Beltramo E, Pagnozzi F, Bena E, Molinatti GM, Porta M (1996) Thiamine corrects delayed replication and decreases production of lactate and advanced glycation end-products in bovine retinal and human umbilical vein endothelial cells cultured under high glucose conditions. Diabetologia 39:1263–1268
Thornalley PJ, Jahan I, Ng R (2001) Suppression of the accumulation of triosephosphates and increased formation of methylglyoxal in human red blood cells during hyperglycaemia by thiamine in vitro. J Biochem (Tokyo) 129:543–549
Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9:294–299
Berrone E, Beltramo E, Solimine C, Ape AU, Porta M (2006) Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem 281:9307–9313
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest 52:2745–2756
Creager MA, Lüscher TF, Cosentino F, Beckman JA (2003) Diabetes and vascular disease: pathology, clinical consequences, and medical therapy. Part I. Circulation 108:1527–1532
Visse R, Nagase H (2003) Matrix metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839
Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T (2003) Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253:269–285
Woessner JF, Nagase H (2000) Protein substrates of the MMPs. In matrix metalloproteinases and TIMPs, 1st edn. Oxford University Press, New York, pp 87–97
Podestá F, Roth T, Ferrara F, Cagliero E, Lorenzi M (1997) Cytoskeletal changes induced by excess extracellular matrix impairs endothelial cell replication. Diabetologia 40:879–886
Jacqueminet S, Ben Abdesselam O, Chapman MJ, Nicolay N, Foglietti MJ, Grimaldi A, Beaudeux JL (2006) Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta 367:103–107
Noda K, Ishida S, Inoue M, Obata K, Oguchi Y, Okada Y, Ikeda E (2003) Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 44:2163–2170
Ishizaki E, Takai S, Ueki M, Maeno T, Maruichi M, Sugiyama T, Oku H, Ikeda T, Miyazaki M (2006) Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol 141:129–134
John A, Tuszynski G (2001) The role of matrix metalloproteinases in tumour angiogenesis and tumour metastasis. Pathol Oncol Res 7:14–23
Corbel M, Belleguic C, Boichot E, Lagente V (2002) Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol 18:51–61
Giebel SJ, Menicucci G, McGuire PG, Das A (2005) Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood–retinal barrier. Lab Invest 85:597–607
Beaudeux JL, Giral P, Bruckert E, Bernard M, Foglietti MJ, Chapman MJ (2003) Serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 as potential markers of carotid atherosclerosis in infraclinical hyperlipidemia. Atherosclerosis 169:139–146
Kuzuya M, Iguchi A (2003) Role of matrix metalloproteinases in vascular remodelling. J Atheroscler Thromb 10:275–282
Barth JL, Yu Y, Song W, Lu K, Dashti A, Huang Y, Argraves WS, Lyons TJ (2007) Oxidised, glycated LDL selectively influences tissue inhibitor of metalloproteinase-3 gene expression and protein production in human retinal capillary pericytes. Diabetologia 50:2200–2208
Grant MB, Caballero S, Tarnuzzer RW, Bass KE, Ljubimov AV, Spoerri PE, Galardy RE (1998) Matrix metalloproteinase expression in human retinal microvascular cells. Diabetes 47:1311–1317
Garner A (1993) Histopathology of diabetic retinopathy in man. Eye 7:250–253
Taylor CM, Thompson JM, Weiss JB (1991) Matrix integrity and the control of angiogenesis. Int J Radiat Biol 60:61–64
Furness PN (1997) Basement membrane synthesis and degradation. J Pathol 183:1–3
Ha H, Lee HB (2005) Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and diabetic kidney. Nephrology (Carlton) S7–S10
Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS (2001) Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res 88:1291–1298
Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS (1996) Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest 98:2572–2579
Ho FM, Liu SH, Lin WW, Liau CS (2007) Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem 101:442–450
Beltramo E, Berrone E, Tarallo S, Porta M (2008) Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol 45:131–141
Pomero F, Molinar Min A, La Selva M, Allione A, Molinatti GM, Porta M (2001) Benfotiamine is similar to thiamine in correcting endothelial cell defects induced by high glucose. Acta Diabetol 38:135–138
Beltramo E, Pomero F, Allione A, D’Alù F, Ponte E, Porta M (2002) Pericyte adhesion is impaired on extracellular matrix produced by endothelial cells in high hexose concentrations. Diabetologia 45:416–419
Beltramo E, Buttiglieri S, Pomero F, Allione A, D’Alù F, Ponte E, Porta M (2003) A study of capillary pericyte viability on extracellular matrix produced by endothelial cells in high glucose. Diabetologia 46:409–415
Acknowledgments
This work was supported by grants from the Compagnia di San Paolo, Turin, Italy. E. Beltramo was the recipient of the 2002 European Association for the Study of Diabetes (EASD)/Eli Lilly Research Fellowship in Diabetes Microvascular Complications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarallo, S., Beltramo, E., Berrone, E. et al. Effects of high glucose and thiamine on the balance between matrix metalloproteinases and their tissue inhibitors in vascular cells. Acta Diabetol 47, 105–111 (2010). https://doi.org/10.1007/s00592-009-0124-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-009-0124-5