Abstract
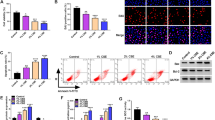
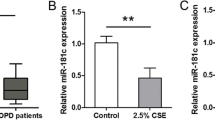
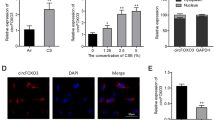
To investigate the effects of Baicalin on the apoptosis of human bronchial epithelial cells (16HBE) induced by cigarette smoke extract (CSE) and the release of inflammatory factors, and to clarify its possible mechanism. CSE was used to treat 16HBE cells and construct COPD cell model. The activity of 16HBE cells was detected by CCK-8 and BrdU. Real-time fluorescence quantitative PCR (RT-QPCR) was used to detect the expression level of miR-125a in each group of 16HBE cells. At the same time, the levels of 16HBE inflammatory cytokines IL-1β, IL-8, IL-6, and TNF-α were detected. The apoptosis rate of 16HBE cells in each group was detected by TUNEL. Compared with the control group, the proliferation of 16HBE cells in CSE group was decreased. Baicalin reversed the effect of 2% CSE on the proliferation of 16HBE cells. Baicalin also reversed the effect of 2% CSE on apoptosis and inflammatory factors in 16HBE cells. miR-125a is highly expressed in COPD, and Baicalin can inhibit the expression of miR-125a. Silencing miR-125a reduces apoptosis and inflammatory response of 16HBE cells in COPD. miR-125a reversed the effects of Baicalin on apoptosis and inflammation of 16HBE cells. Baicalin can reduce CSE-induced apoptosis of human bronchial epithelial cells and release of inflammatory factors, and its mechanism may be related to the inhibition of miR-125a.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Wang, Y., et al. (2018). Role of inflammatory cells in airway remodeling in COPD. International journal of chronic obstructive pulmonary disease, 13, 3341.
de Llano, L. P., et al. (2018). Mixed Th2 and non-Th2 inflammatory pattern in the asthma–COPD overlap: A network approach. International journal of chronic obstructive pulmonary disease, 13, 591.
Huckle, A. W., Fairclough, L. C., & Todd, I. (2018). Prophylactic antibiotic use in COPD and the potential anti-inflammatory activities of antibiotics. Respiratory care, 63(5), 609–619.
Atto, B., et al. (2019). New therapeutic targets for the prevention of infectious acute exacerbations of COPD: Role of epithelial adhesion molecules and inflammatory pathways. Clinical Science, 133(14), 1663–1703.
Cui, W., et al. (2018). Nrf2 attenuates inflammatory response in COPD/emphysema: Crosstalk with Wnt3a/β-catenin and AMPK pathways. Journal of Cellular and Molecular Medicine, 22(7), 3514–3525.
Yang, Y., et al., (2020). Advances in pharmacological actions and mechanisms of flavonoids from traditional Chinese medicine in treating chronic obstructive pulmonary disease. Evidence-Based Complementary and Alternative Medicine, 2020.
Mohamed, A., et al. (2019). Polymeric nanoparticles for the delivery of miRNA to treat chronic obstructive pulmonary disease (COPD). European Journal of Pharmaceutics and Biopharmaceutics, 136, 1–8.
Asensio, V. J., et al. (2020). Eosinophilic COPD patients display a distinctive serum miRNA profile from asthma and non-eosinophilic COPD. Archivos de Bronconeumología (English Edition), 56(4), 234–241.
Chen, J., et al. (2020). Whole transcriptome-based miRNA-mRNA network analysis revealed the mechanism of inflammation-immunosuppressive damage caused by cadmium in common carp spleens. Science of The Total Environment, 717, 137081.
An, J., et al. (2021). Identifying miRNA modules and related pathways of chronic obstructive pulmonary disease associated emphysema by weighted gene co-expression network analysis. International journal of chronic obstructive pulmonary disease, 16, 3119.
Kuatama, R., Jusni, L. F. J., & Karina, C. (2021). A review of miRNAs accuracy as a diagnostic biomarker in COPD patients. Biomolecular and Health Science Journal, 4(1), 61–65.
Guo, S., et al. (2010). MicroRNA miR-125a controls hematopoietic stem cell number. Proceedings of the National Academy of Sciences, 107(32), 14229–14234.
Jiang, L., et al. (2010). Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer, 10(1), 1–13.
Kim, S.-W., et al. (2012). MicroRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proceedings of the National Academy of Sciences, 109(20), 7865–7870.
Hu, H.-L., et al., (2017). Circulating miR-125b but not miR-125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine, 96(51).
李倩, et al., (2023). 中药黄芩抑菌作用研究进展. 西部中医药, 36(02): 137–140.
Shieh, D.-E., Liu, L.-T., & Lin, C.-C. (2000). Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer research, 20(5A), 2861–2865.
Huang, T., Liu, Y., & Zhang, C. (2019). Pharmacokinetics and bioavailability enhancement of baicalin: A review. European Journal of Drug Metabolism and Pharmacokinetics, 44(2), 159–168.
Fang, P., et al. (2020). Baicalin and its aglycone: A novel approach for treatment of metabolic disorders. Pharmacological Reports, 72(1), 13–23.
Lixuan, Z., et al. (2010). Baicalin attenuates inflammation by inhibiting NF-κB activation in cigarette smoke induced inflammatory models. Pulmonary pharmacology & therapeutics, 23(5), 411–419.
Wang, G., et al., (2018). Baicalin exerts anti-airway inflammation and anti-remodelling effects in severe stage rat model of chronic obstructive pulmonary disease. Evidence-Based Complementary and Alternative Medicine, 2018.
Hao, D., et al. (2021). Baicalin alleviates chronic obstructive pulmonary disease through regulation of HSP72-mediated JNK pathway. Molecular Medicine, 27(1), 53.
Zhang, H., et al. (2021). Baicalin ameliorates cigarette smoke-induced airway inflammation in rats by modulating HDAC2/NF-κB/PAI-1 signalling. Pulmonary Pharmacology & Therapeutics, 70, 102061.
Rodriguez-Roisin, R. (2000). Toward a consensus definition for COPD exacerbations. Chest, 117(5), 398S-401S.
Mannino, D. M. (2002). COPD: Epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest, 121(5), 121S-126S.
Bartal, M., (2005). COPD and tobacco smoke. Monaldi archives for chest disease. 63(4).
Taylor, J. D. (2010). COPD and the response of the lung to tobacco smoke exposure. Pulmonary pharmacology & therapeutics, 23(5), 376–383.
Leberl, M., Kratzer, A., & Taraseviciene-Stewart, L. (2013). Tobacco smoke induced COPD/emphysema in the animal model—are we all on the same page? Frontiers in physiology, 4, 91.
Camp, P. G., et al. (2014). COPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican women. European Respiratory Journal, 43(3), 725–734.
Olloquequi, J., et al. (2018). Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respiratory research, 19(1), 1–8.
Kim, W. J., & Lee, C. Y. (2017). Environmental exposures and chronic obstructive pulmonary disease. Molecular & Cellular Toxicology, 13(3), 251–255.
Hashiguchi, Y., et al. (2012). Down-regulation of miR-125a-3p in human gastric cancer and its clinicopathological significance. International journal of oncology, 40(5), 1477–1482.
Sheng, W., et al. (2022). Curcumol inhibits the development of prostate cancer by miR-125a/STAT3 Axis. Evid Based Complement Alternat Med, 2022, 9317402.
Ahmadpour, F., et al. (2022). Methylation-mediated silencing of miR-125a-5p facilitates breast cancer progression by inducing autophagy. Molecular Biology Reports, 49(7), 6325–6339.
Tong, Y., et al. (2022). LncRNA TP73-AS1 exacerbates the non-small-cell lung cancer (NSCLC) process via regulating miR-125a-3p-mediated ACTN4. Evid Based Complement Alternat Med, 2022, 4098271.
Zhu, J., Wang, W., & Wu, X. (2020). Isorhynchophylline exerts anti-asthma effects in mice by inhibiting the proliferation of airway smooth muscle cells: The involvement of miR-200a-mediated FOXC1/NF-κB pathway. Biochemical and Biophysical Research Communications, 521(4), 1055–1060.
Singh, S., Meena, A., & Luqman, S. (2021). Baicalin mediated regulation of key signaling pathways in cancer. Pharmacological Research, 164, 105387.
Wang, G., et al. (2018). Baicalin exerts anti-airway inflammation and anti-remodelling effects in severe stage rat model of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med, 2018, 7591348.
Hu, L.A.-O., et al. Baicalin inhibits airway smooth muscle cells proliferation through the RAS signaling pathway in murine asthmatic airway remodeling model, Electronic (pp. 1942–0994).
Funding
This work was supported by the General Program of The National Natural Science Foundation of China (81470240), the Scientific Research Project of the Shanxi Provincial Health Commission (201601010), the Doctoral Foundation of Shanxi Bethune Hospital (2019YJ01), and the Fundamental Research Program of Shanxi Province (20210302123492).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
It is not applicable.
Informed Consent
It is not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, X., Huo, J., Li, L. et al. Baicalin Relieves Airway Inflammation in COPD by Inhibiting miR-125a. Appl Biochem Biotechnol (2023). https://doi.org/10.1007/s12010-023-04671-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04671-y