Abstract
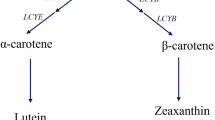
Aurantiochytrium limacinum is a heterotrophic eukaryotic microorganism that can accumulate high levels of commercial products such as astaxanthin and docosahexaenoic acid. Due to its rapid growth and relatively simple extraction method, A. limacinum is considered a promising astaxanthin resource to replace the conventional microalgal production. However, the astaxanthin biosynthetic process in A. limacinum remains incompletely understood, especially in those catalysed by β-carotene hydroxylase (CrtZ) and ketolase. In this study, we overexpressed a crtZ candidate gene to increase astaxanthin production and expand our understanding of the conversion from beta-carotene to astaxanthin. The resultant transformant AlcrtZ#10 cultivated for 5 days showed a significant increase in astaxanthin production per culture (2.8-fold) and per cell (4.5-fold) compared with that of the wild-type strain. Strikingly, longer light exposure increased astaxanthin production and decreased the beta-carotene content in the wild-type strain, suggesting that light exposure duration is important for astaxanthin production in A. limacinum. Among several predicted intermediates, furthermore, the cantaxanthin produced from β-carotene by ketolase activity were enhanced in the transformant AlcrtZ#10. Although the further investigation is needed, this result suggested that the main route of astaxanthin was via cantaxanthin. Thus, our findings will be valuable not only for its application, but also for understanding the astaxanthin biosynthetic process in A. limacinum.






Similar content being viewed by others
References
Iida, I., Nakahara, T., Yokochi, T., Kamisaka, Y., Yagi, H., Yamaoka, M., & Suzuki, O. (1996). Improvement of docosahexaenoic acid production in a culture of Thraustochytrium aureum by medium optimization. Journal of Fermentation and Bioengineering, 81, 76–78.
Mozaffarian, D., & Wu, J. H. Y. (2011). Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology, 58, 2047–2067.
Calder, P. C. (2006). n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition, 83, 1505S-1519S.
Birch, E. E., Hoffman, D. R., Uauy, R., Birch, D. G., & Prestidge, C. (1998). Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatric Research, 44, 201–209.
Innis, S. M. (2007). Dietary (n-3) fatty acids and brain development. Journal of Nutrition, 137, 855–859.
Horrocks, L. A., & Yeo, Y. K. (1999). Health benefits of docosahexaenoic acid (DHA). Pharmacological Research, 40, 211–225.
Lewis, T. E., Nichols, P. D., & McMeekin, T. A. (1999). The biotechnological potential of thraustochytrids. Marine Biotechnology, 1, 580–587.
Yokochi, T., Honda, D., Higashihara, T., & Nakahara, T. (1998). Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Applied Microbiology and Biotechnology, 49, 72–76.
Barclay, W., Weaver, C., Metz, J., & Hansen, J. (2010). Development of a docosahexaenoic acid production technology using Schizochytrium: Historical perspective and update. In Z. Cohen, & C. Ratledge (Eds.), Single cell oils: Microbial and algal oils (2nd ed., pp. 75–96). AOCS.
Weete, J. D., Kim, H., Gandhi, S. R., Wang, Y., & Dute, R. (1997). Lipids and ultrastructure of Thraustochytrium sp. ATCC 26185. Lipids, 32, 839–845.
Ramos-Vega, A., Rosales-Mendoza, S., Bañuelos-Hernández, B., & Angulo, C. (2018). Prospects on the use of Schizochytrium sp. to develop oral vaccines. Frontiers in Microbiology, 9, 2506.
Bagul, V. P., & Annapure, U. S. (2020). Effect of sequential recycling of spent media wastewater on docosahexaenoic acid production by newly isolated strain Aurantiochytrium sp. ICTFD5. Bioresource Technology, 306, 123153.
Humaidah, N., Nakai, S., Nishijima, W., Gotoh, T., & Furuta, M. (2020). Application of Aurantiochytrium sp. L3W for food-processing wastewater treatment in combination with polyunsaturated fatty acids production for fish aquaculture. Science of the Total Environment, 743, 140735.
Yamasaki, T., Aki, T., Shinozaki, M., Taguchi, M., Kawamoto, S., & Ono, K. (2006). Utilization of Shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. Journal of Bioscience and Bioengineering, 102, 323–327.
Aki, T., Hachida, K., Yoshinaga, M., Katai, Y., Yamasaki, T., Kawamoto, S., … Ono, K. (2003). Thraustochytrid as a potential source of carotenoids. JAOCS, Journal of the American Oil Chemists’ Society, 80, 789–794.
Yokoyama, R., & Honda, D. (2007). Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): Emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience, 48, 199–211.
Kubo, Y., Shiroi, M., Higashine, T., Mori, Y., Morimoto, D., Nakagawa, S., & Sawayama, S. (2021). Enhanced production of astaxanthin without decrease of DHA content in Aurantiochytrium limacinum by overexpressing multifunctional carotenoid synthase gene. Applied Biochemistry and Biotechnology, 193, 52–64.
Demmig-Adams, B. (1990). Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. BBA - Bioenergetics, 1020, 1–24. https://doi.org/10.1016/0005-2728(90)90088-L
Gul, K., Tak, A., Singh, A. K., Singh, P., Yousuf, B., & Wani, A. A. (2015). Chemistry, encapsulation, and health benefits of β-carotene - A review. Cogent Food & Agriculture, 1, 1018696.
Nishida, Y., Yamashita, E., & Miki, W. (2007). Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Science, 11, 16–20.
Guerin, M., Huntley, M. E., & Olaizola, M. (2003). Haematococcus astaxanthin: Applications for human health and nutrition. Trends in Biotechnology, 21, 210–216.
Berman, J., Zorrilla-López, U., Farré, G., Zhu, C., Sandmann, G., Twyman, R. M., … Christou, P. (2015). Nutritionally important carotenoids as consumer products. Phytochemistry Reviews, 14, 727–743.
Novoveská, L., Ross, M. E., Stanley, M. S., Pradelles, R., Wasiolek, V., & Sassi, J. F. (2019). Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Marine Drugs, 17, 640.
Zhang, C., Chen, X., & Too, H. P. (2020). Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Applied Microbiology and Biotechnology, 104, 5725–5737.
Guimarães, A. M., Dias Schleder, D., Nagata, M., Nóbrega, R. O., Fracalossi, D. M., Quadros Seiffert, W., & do Nascimento Vieira, F. (2019). Aurantiochytrium sp. meal can replace fish oil in practical diets for the juvenile Pacific white shrimp. Aquaculture Nutrition, 25, 798–807.
Moran, C. A., Morlacchini, M., Keegan, J. D., & Fusconi, G. (2018). The effect of dietary supplementation with Aurantiochytrium limacinum on lactating dairy cows in terms of animal health, productivity and milk composition. Journal of Animal Physiology and Animal Nutrition, 102, 576–590.
Nobrega, R. O., Batista, R. O., Corrêa, C. F., Mattioni, B., Filer, K., Pettigrew, J. E., & Fracalossi, D. M. (2019). Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture, 507, 500–509.
Liu, B., Jiang, J., Yu, D., Lin, G., & Xiong, Y. L. (2020). Effects of supplementation of microalgae (Aurantiochytrium sp.) to laying hen diets on fatty acid content, health lipid indices, oxidative stability, and quality attributes of meat. Foods, 9, 1271.
Furlan, V. J. M., Batista, I., Bandarra, N., Mendes, R., & Cardoso, C. (2019). Conditions for the production of carotenoids by Thraustochytrium sp. ATCC 26185 and Aurantiochytrium sp. ATCC PRA-276. Journal of Aquatic Food Product Technology, 28, 465–477.
Lee Chang, K. J., Dunstan, G. A., Abell, G. C. J., Clementson, L. A., Blackburn, S. I., Nichols, P. D., & Koutoulis, A. (2012). Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Applied Microbiology and Biotechnology, 93, 2215–2231.
Watanabe, K., Arafiles, K. H. V., Higashi, R., Okamura, Y., Tajima, T., Matsumura, Y., … Aki, T. (2018). Isolation of high carotenoid-producing Aurantiochytrium sp. mutants and improvement of astaxanthin productivity using metabolic information. Journal of Oleo Science, 67, 571–578.
Suen, Y. L., Tang, H., Huang, J., & Chen, F. (2014). Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. Journal of Agricultural and Food Chemistry, 62, 12392–12398.
Iwasaka, H., Koyanagi, R., Satoh, R., Nagano, A., Watanabe, K., Hisata, K., … Aki, T. (2018). A possible trifunctional β-carotene synthase gene identified in the draft genome of Aurantiochytrium sp. Strain KH105. Genes, 9, 200.
Misawa, N., Satomi, Y., Kondo, K., Yokoyama, A., Kajiwara, S., Saito, T., … Miki, W. (1995). Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. Journal of Bacteriology, 177, 6575–6584.
Dellero, Y., Cagnac, O., Rose, S., Seddiki, K., Cussac, M., Morabito, C., … Amato, A. (2018). Proposal of a new thraustochytrid genus Hondaea gen. nov. and comparison of its lipid dynamics with the closely related pseudo-cryptic genus Aurantiochytrium. Algal Research, 35, 125–141.
Adachi, T., Sahara, T., Okuyama, H., & Morita, N. (2017). Glass bead-based genetic transformation: An efficient method for transformation of thraustochytrid microorganisms. Journal of Oleo Science, 66, 791–795.
Sakaguchi, K., Matsuda, T., Kobayashi, T., Ohara, J. I., Hamaguchi, R., Abe, E., … Ito, M. (2012). Versatile transformation system that is applicable to both multiple transgene expression and gene targeting for thraustochytrids. Applied and Environmental Microbiology, 78, 3193–3202.
Lin, Y., Xie, X., Yuan, B., Fu, J., Liu, L., Tian, H., … He, D. (2018). Optimization of enzymatic cell disruption for improving lipid extraction from Schizochytrium sp. through response surface methodology. Journal of Oleo Science, 67, 215–224.
Kubo, Y., Morimoto, D., Shiroi, M., Yoshimi, T., Ohara, K., Higashine, T., … Sawayama, S. (2022). Transcriptional responses of Aurantiochytrium limacinum under light conditions. Journal of Applied Microbiology, 132(6).
Gong, Z., Wang, H., Tang, J., Bi, C., Li, Q., & Zhang, X. (2020). Coordinated expression of astaxanthin biosynthesis genes for improved astaxanthin production in Escherichia coli. Journal of Agricultural and Food Chemistry, 68, 14917–14927.
Römer, S., Fraser, P. D., Kiano, J. W., Shipton, C. A., Misawa, N., Schuch, W., & Bramley, P. M. (2000). Elevation of the provitamin A content of transgenic tomato plants. Nature Biotechnology, 18, 666–669.
Hwang, K. S., Kim, H. U., Charusanti, P., Palsson, B. T., & Lee, S. Y. (2014). Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnology Advances, 32, 255–268.
Chen, G., Wang, B., Han, D., Sommerfeld, M., Lu, Y., Chen, F., & Hu, Q. (2015). Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant Journal, 81, 95–107.
Huang, W., Ye, J., Zhang, J., Lin, Y., He, M., & Huang, J. (2016). Transcriptome analysis of Chlorella zofingiensis to identify genes and their expressions involved in astaxanthin and triacylglycerol biosynthesis. Algal Research, 17, 236–243.
Ye, J., Liu, M., He, M., Ye, Y., & Huang, J. (2019). Illustrating and enhancing the biosynthesis of astaxanthin and docosahexaenoic acid in Aurantiochytrium sp. SK4. Marine Drugs, 17, 45. https://doi.org/10.3390/md17010045
Converti, A., Casazza, A. A., Ortiz, E. Y., Perego, P., & Del Borghi, M. (2009). Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chemical Engineering and Processing: Process Intensification, 48, 1146–1151.
Li, Y., Han, D., Hu, G., Sommerfeld, M., & Hu, Q. (2010). Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnology and Bioengineering, 107, 258–268.
Choi, S. K., Harada, H., Matsuda, S., & Misawa, N. (2007). Characterization of two β-carotene ketolases, CrtO and CrtW, by complementation analysis in Escherichia coli. Applied Microbiology and Biotechnology, 75, 1335–1341.
Funding
This work was supported by Grants-in Aids for Scientific Research (B) (No. 22H02237) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analyses were performed by Toru Yoshimi and Sakiko Hashimoto. The first draft of the manuscript was written by Toru Yoshimi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

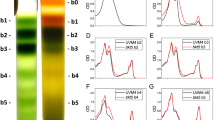
Fig. S1
Agarose gel electrophoresis of PCR products amplified from transformed A. limacinum colonies. Genomic integration of pAlcrtZ was verified by colony PCR of G418-resistant A. limacinum colonies transformed with pAlcrtZ (AlcrtZ#4, 7, 10, 13, 14). Arrow indicates PCR products amplified from pAlcrtZ using the PCR primers pAl-Neo-F and pAl-Ter-R.

Fig. S2
HPLC chromatogram of carotenoids extracted from transformant AlcrtZ#10 on day 5 (using 6-fold concentrated extract). 1, Astaxanthin; 2, Canthaxanthin; 3, β-carotene
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshimi, T., Hashimoto, S., Kubo, Y. et al. Improvement of Astaxanthin Production in Aurantiochytrium limacinum by Overexpression of the Beta-Carotene Hydroxylase Gene. Appl Biochem Biotechnol 195, 1255–1267 (2023). https://doi.org/10.1007/s12010-022-04172-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04172-4