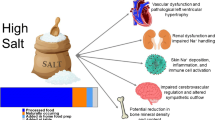
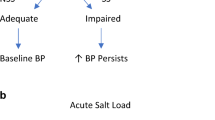
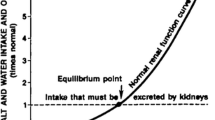
Abstract
Purpose of Review
To review underlying mechanisms and environmental factors that may influence racial disparities in the development of salt-sensitive blood pressure.
Recent Findings
Our group and others have observed racial differences in diet and hydration, which may influence salt sensitivity. Dietary salt elicits negative alterations to the gut microbiota and immune system, which may increase hypertension risk, but little is known regarding potential racial differences in these physiological responses. Antioxidant supplementation and exercise offset vascular dysfunction following dietary salt, including in Black adults. Furthermore, recent work proposes the role of racial differences in exposure to social determinants of health, and differences in health behaviors that may influence risk of salt sensitivity.
Summary
Physiological and environmental factors contribute to the mechanisms that manifest in racial differences in salt-sensitive blood pressure. Using this information, additional work is needed to develop strategies that can attenuate racial disparities in salt-sensitive blood pressure.




Similar content being viewed by others
Abbreviations
- ARIC:
-
Atherosclerosis risk in communities
- BP:
-
Blood pressure
- CV:
-
Cardiovascular
- CRP:
-
C-reactive protein
- DASH:
-
Dietary Approaches to Stop Hypertension
- ENaC:
-
Epithelial sodium channel
- H2O2 :
-
Hydrogen peroxide
- HUVECs:
-
Human umbilical vein endothelial cells
- IL-6:
-
Interleukin-6
- META-Health:
-
Morehouse and Emory Team up to Eliminate Health Disparities
- MESA:
-
Multi-ethnic study of atherosclerosis
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NHANES:
-
National Health and Nutrition Examination Survey
- NGAL:
-
Neutrophil gelatinase–associated lipocalin
- NO:
-
Nitric oxide
- ONOO– :
-
Peroxynitrate
- O2 − :
-
Superoxide
- RAAS:
-
Renin-angiotensin-aldosterone system
- REGARDS:
-
Reasons for Geographic and Racial Differences in Stroke
- ROS:
-
Reactive oxygen species
- SDoH:
-
Social determinants of health
- SCFA:
-
Short-chain fatty acid
- SGK1:
-
Serum and glucocorticoid-regulated kinase 1
- SOD:
-
Superoxide dismutase
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans. 2020–2025.
Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–92.
Tsao CW, et al. Heart disease and stroke statistics-2023 update: a report from the american heart association. Circulation. 2023;147(8):e93–621.
Xu J, et al. Mortality in the United States, 2021. NCHS Data Brief. 2022;456:1–8.
Elijovich F, et al. Salt sensitivity of blood pressure. Hypertension. 2016;68(3):e7–46.
Barba G, et al. Incidence of hypertension in individuals with different blood pressure salt-sensitivity: results of a 15-year follow-up study. J Hypertens. 2007;25(7):1465–71.
Morimoto A, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350(9093):1734–7.
Weinberger MH, et al. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Pt 2):429–32.
• Linder BA, et al. Short-term high salt intake does not influence resting or exercising heart rate variability but increases MCP-1 concentration in healthy young adults. Am J Physiol Regul Integr Comp Physiol. 2023;324(5):R666–76. This paper from our group highlights the influence of short-term high dietary salt on an inflammatory biomarker, plasma monocyte chemoattractant protein 1.
Barnett AM, et al. High dietary salt intake increases urinary NGAL excretion and creatinine clearance in healthy young adults. Am J Physiol Renal Physiol. 2022.
Babcock MC, et al. High salt intake augments blood pressure responses during submaximal aerobic exercise. J Am Heart Assoc. 2020;9(10).
Overlack A, et al. Age is a major determinant of the divergent blood pressure responses to varying salt intake in essential hypertension. Am J Hypertens. 1995;8(8):829–36.
Dengel DR, et al. Effect of aerobic exercise training on blood pressure sensitivity to dietary sodium in older hypertensives. J Hum Hypertens. 2006;20(5):372–8.
Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in Black Americans. Hypertension. 1996;28(5):854–8.
Elijovich F, et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68(3):e7–46.
Sacks FM, DASH-Sodium Collaborative Research Group, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10.
Vollmer WM, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135(12):1019–28.
Morris RC, et al. Normotensive salt sensitivity. Hypertension. 1999;33(1):18–23.
Schmidlin O, et al. NaCl-induced renal vasoconstriction in salt-sensitive African Americans. Hypertension. 1999;33(2):633–9.
• Hunter SD, Kavouras SA, Rahimi M. Exploring heated exercise as a means of preventing the deleterious effects of high-sodium intake in Black women. Am J Physiol Heart Circ Physiol. 2023;324(6):H833-h839. This paper highlights the influence of exercise, specifically hot yoga, and high dietary salt on endothelial function in Black female adults.
Luft FC, et al. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation. 1979;60(3):697–706.
Dimsdale JE, et al. Prediction of salt sensitivity. Am J Hypertens. 1990;3(6 Pt 1):429–35.
Robinson AT, Wenner MM, Charkoudian N. Differential influences of dietary sodium on blood pressure regulation based on race and sex. Auton Neurosci. 2021;236:102873.
Sudhir K, et al. Reduced dietary potassium reversibly enhances vasopressor response to stress in African Americans. Hypertension. 1997;29(5):1083–90.
He FJ, et al. Importance of the renin system in determining blood pressure fall with salt restriction in Black and White hypertensives. Hypertension. 1998;32(5):820–4.
Falkner B, Kushner H. Effect of chronic sodium loading on cardiovascular response in young Blacks and Whites. Hypertension. 1990;15(1):36–43.
JT Wright, et al. Determinants of salt sensitivity in Black and White normotensive and hypertensive women. Hypertension (Dallas, Tex. : 1979). 2003;42(6).
Appel LJ, et al. Effects of reduced sodium intake on hypertension control in older individuals. Arch Intern Med. 2001;161(5):685.
Palacios C, et al. Sodium retention in Black and White female adolescents in response to salt intake. J Clin Endocrinol Metab. 2004;89(4):1858–63.
He FJ, et al. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in White, Black, and Asian mild hypertensives. Hypertension. 2009;54(3):482–8.
Kurtz TW, et al. No evidence of racial disparities in blood pressure salt sensitivity when potassium intake exceeds levels recommended in the US dietary guidelines. Am J Physiol Heart Circ Physiol. 2021;320(5):H1903-h1918.
• Ferguson JF, et al. High dietary salt–induced DC activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;4(13). This paper observed immune cell activation induced by high dietary salt and its association with gut dysbiosis in humans and mice.
Nichols OI, et al. Neighborhood socioeconomic deprivation in early childhood mediates racial disparities in blood pressure in a college student sample. J Youth Adolesc. 2022.
Grosicki GJ, et al. Acute beetroot juice reduces blood pressure in young Black and White males but not females. Redox Biol. 2023;63:102718.
Voors AW, et al. Studies of blood pressures in children, ages 5–14 years, in a total biracial community: the Bogalusa Heart Study. Circulation. 1976;54(2):319–27.
• Caraballo C, et al. Excess mortality and years of potential life lost among the Black Population in the US, 1999–2020. JAMA. 2023;329(19):1662–70. This paper reported data from the Centers for Disease Control over the recent 22-year period comparing excess death and years of life lost between the Black and White populations.
Rao S, et al. Association of genetic West African ancestry, blood pressure response to therapy, and cardiovascular risk among self-reported Black individuals in the systolic blood pressure reduction intervention trial (SPRINT). JAMA Cardiol. 2020.
Haas AV, et al. Genetic predictors of salt sensitivity of blood pressure: the additive impact of 2 hits in the same biological pathway. Hypertension. 2021;78(6):1809–17.
Grosicki GJ, et al. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: social determinants of health. Prog Cardiovasc Dis. 2022;71:4–10.
Robinson AT, Cook MD, Lane-Cordova AD. Making cell culture more physiological: a call for more a comprehensive assessment of racial disparities in endothelial cell culture studies. Am J Physiol Cell Physiol. 2019.
Kirkman D, et al. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness and cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2021.
Green DJ, et al. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–9.
Golia E, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16(9).
Sessa WC. eNOS at a glance. J Cell Sci. 2004;117(Pt 12):2427–9.
Cook NR, et al. Sodium and health-concordance and controversy. BMJ. 2020;369:m2440.
Watso JC, et al. The damaging duo: obesity and excess dietary salt contribute to hypertension and cardiovascular disease. Obes Rev. 2023;24(8):e13589.
Robinson AT, Edwards DG, Farquhar WB. The influence of dietary salt beyond blood pressure. Curr Hypertens Rep. 2019;21(6):42.
Patik JC, et al. Mechanisms of dietary sodium-induced impairments in endothelial function and potential countermeasures. Nutrients. 2021;13(1):270.
Greaney JL, et al. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590(21):5519–28.
Guers JJ, et al. Voluntary wheel running prevents salt-induced endothelial dysfunction: role of oxidative stress. J Appl Physiol (1985). 2018.
Durand MJ, Lombard JH. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt–fed rats by increasing copper/zinc superoxide dimutase expression. Am J Hypertens. 2013;26(6):739–47.
Robinson AT, et al. Microvascular vasodilator plasticity after acute exercise. Exerc Sport Sci Rev. 2018;46(1):48–55.
• Decker KP, et al. High sodium intake differentially impacts brachial artery dilation when evaluated with reactive versus active hyperemia in salt resistant individuals. J Appl Physiol (1985). 2023;134(2): 277–87. This paper demonstrated impaired brachial artery flow–mediated dilation after high dietary salt during reactive hyperemia, but this effect was reversed by acute antioxidant supplementation.
Ramick MG, et al. Apocynin and Tempol ameliorate dietary sodium-induced declines in cutaneous microvascular function in salt-resistant humans. Am J Physiol Heart Circ Physiol. 2019;317(1):H97-h103.
Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;317(4):H777-h789.
Bunsawat K, et al. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: physiological underpinnings. Prog Cardiovasc Dis. 2022;71:11–9.
Heffernan KS, et al. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol. 2008;295(6):H2380–7.
D’Agata MN, et al. Young black women demonstrate impaired microvascular but preserved macrovascular function compared to white women. Exp Physiol. 2021;106(10):2031–7.
D’Agata MN, et al. Evidence of reduced peripheral microvascular function in young Black women across the menstrual cycle. J Appl Physiol (1985). 2021;131(6):1783–91.
Morris AA, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory team up to eliminate health disparities (META-Health) study. Metab Syndr Relat Disord. 2012;10(4):252–9.
Feairheller DL, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4(1):32–7.
Plante TB, et al. C-reactive protein and incident hypertension in Black and White Americans in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study. Am J Hypertens. 2021;34(7):698–706.
Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation. 2016;133(9):894–906.
Mutchler SM, Kirabo A, Kleyman TR. Epithelial sodium channel and salt-sensitive hypertension. Hypertension. 2021;77(3):759–67.
Elijovich F, et al. Immune mechanisms of dietary salt-induced hypertension and kidney disease: Harry Goldblatt Award for early career investigators 2020. Hypertension. 2021;78(2):252–60.
Ruggeri Barbaro N, et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res. 2021;117(5):1358–71.
Zaika O, et al. Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr Hypertens Rep. 2013;15(1):17–24.
Morris RC, et al. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;133(9):881–93.
Schmidlin O, Sebastian AF, Morris RC Jr. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49(5):1032–9.
Schmidlin OFA, Tanaka M, Sebastian A, Morris RC. NaCl-induced renal vasoconstriction in salt-sensitive African Americans: antipressor and hemodynamic effects of potassium bicarbonate. Hypertension (Dallas, Tex. : 1979). 1999;33(2).
Fink GD, et al. Determinants of renal vascular resistance in the Dahl strain of genetically hypertensive rat. Hypertension. 1980;2(3):274–80.
Wang W, Chonchol M, Seals DR, Nowak KL. Dietary sodium restriction decreases urinary NGAL in older adults with moderately elevated systolic blood pressure free from chronic kidney disease. J Investig Med. 2020;68(7):1271–5.
Pratt JH, et al. Effect of administered potassium on the renin-aldosterone axis in young Blacks compared with Whites. J Hypertens. 1997;15(8):877–83.
Cogswell ME, et al. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319(12):1209.
Lujan HL, DiCarlo SE. The “African gene” theory: it is time to stop teaching and promoting the slavery hypertension hypothesis. Adv Physiol Educ. 2018;42(3):412–6.
Jackson FL. An evolutionary perspective on salt, hypertension, and human genetic variability. Hypertension. 1991;17(1 Suppl):I129–32.
Grollman A. A conjecture about the prevalence of essential hypertension and its high incidence in the Black. Tex Rep Biol Med. 1978;36:25–32.
Wilson TW, Grim CE. Biohistory of slavery and blood pressure differences in Blacks today. A hypothesis. Hypertension. 1991;17(1 Suppl):I122–8.
Wilson TW, Hollifield LR, Grim CE. Systolic blood pressure levels in Black populations in sub-Sahara Africa, the West Indies, and the United States: a meta-analysis. Hypertension. 1991;18(3 Suppl):I87-91.
Poulter NR, Khaw KT, Sever PS. Higher blood pressures of urban migrants from an African low-blood pressure population are not due to selective migration. Am J Hypertens. 1988;1(3 Pt 3):143s–5s.
Curtin PD. The slavery hypothesis for hypertension among African Americans: the historical evidence. Am J Public Health. 1992;82(12):1681–6.
Schmidlin O, et al. Salt sensitivity in Blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58(3):380–5.
Afzaal M, et al. Human gut microbiota in health and disease: unveiling the relationship. Front Microbiol. 2022;13:999001.
Grosicki GJ, et al. Gut check: unveiling the influence of acute exercise on the gut microbiota. Exp Physiol. 2023.
Yang T, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40.
Mell B, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–97.
Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715.
Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5(2):202–7.
Wu Y, et al. The role of short-chain fatty acids of gut microbiota origin in hypertension. Front Microbiol. 2021;12:730809.
• Pluznick JL, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci. 2013;110(11):4410–5. This paper highlights the mechanisms of receptors and short-chain fatty acids in the gut microbiota on blood pressure regulation.
Tilves C, et al. Increases in circulating and fecal butyrate are associated with reduced blood pressure and hypertension: results from the SPIRIT trial. J Am Heart Assoc. 2022;11(13):e024763.
Bier A, et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 2018;10(9):1154.
Myint H, et al. Functional modulation of caecal fermentation and microbiota in rat by feeding bean husk as a dietary fibre supplement. Beneficial Microbes. 2018;9(6):963–74.
Zhai X, et al. Effects of dietary fiber supplementation on fatty acid metabolism and intestinal microbiota diversity in C57BL/6J mice fed with a high-fat diet. J Agric Food Chem. 2018;66(48):12706–18.
Jama HA, et al. Prebiotic intervention with HAMSAB in untreated essential hypertensive patients assessed in a phase II randomized trial. Nature Cardiovasc Res. 2023.
Kaye DM, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141(17):1393–403.
Brooks AW, et al. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16(12):e2006842.
Walejko JM, et al. Gut microbiota and serum metabolite differences in African Americans and White Americans with high blood pressure. Int J Cardiol. 2018;271:336–9.
Carson TL, et al. Associations between race, perceived psychological stress, and the gut microbiota in a sample of generally healthy Black and White women: a pilot study on the role of race and perceived psychological stress. Psychosom Med. 2018;80(7):640–8.
• Hester CM, et al. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21(9):2759–69. This paper revealed that African Americans had lower levels of short-chain fatty acids and higher Firmicute/Bacteroidetes ratio compared with White Americans.
Rothschild D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5.
Ou J, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98(1):111–20.
Zakharia F, et al. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10(12):R141.
• Dugas LR, et al. Decreased microbial co-occurrence network stability and SCFA receptor level correlates with obesity in African-origin women. Sci Rep. 2018;8(1). This paper found that females from Ghana consumed significantly greater dietary fiber, microbial diversity, and short-chain fatty acids compared with females from the U.S.
Price CA, et al. Differences in gut microbiome by insulin sensitivity status in Black and White women of the National Growth and Health Study (NGHS): a pilot study. PLoS ONE. 2022;17(1):e0259889.
Storey M, Anderson P. Income and race/ethnicity influence dietary fiber intake and vegetable consumption. Nutr Res. 2014;34(10):844–50.
Marmot M, et al. Closing the gap in a generation: health equity through action on the social determinants of health. The Lancet. 2008;372(9650):1661–9.
Havranek EP, et al. Social determinants of risk and outcomes for cardiovascular disease. Circulation. 2015;132(9):873–98.
Kucharska-Newton AM, et al. Socioeconomic indicators and the risk of acute coronary heart disease events: comparison of population-based data from the United States and Finland. Ann Epidemiol. 2011;21(8):572–9.
Commodore-Mensah Y, et al. Associations between social determinants and hypertension, stage 2 hypertension, and controlled blood pressure among men and women in the United States. Am J Hypertens. 2021;34(7):707–17.
Roux AVD, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106.
Kaiser P, et al. Neighborhood environments and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2016;183(11):988–97.
Mujahid MS, et al. Historical redlining and cardiovascular health: the multi-ethnic study of atherosclerosis. Proc Natl Acad Sci. 2021;118(51):e2110986118.
Ifatunji MA, et al. Black nativity and health disparities: a research paradigm for understanding the social determinants of health. Int J Environ Res Public Health. 2022;19(15):9166.
Williams DR, et al. Perceived discrimination and psychological well-being in the USA and South Africa. Ethn Health. 2012;17(1–2):111–33.
Mujahid MS, et al. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24(2):187–93.
Forde AT, et al. Discrimination and hypertension risk among African Americans in the Jackson Heart Study. Hypertension. 2020;76(3):715–23.
Koepke JP, Jones S, Dibona GF. Stress increases renal nerve activity and decreases sodium excretion in Dahl rats. Hypertension. 1988;11(4):334–8.
Deter HC, et al. Psychophysiological reactivity of salt-sensitive normotensive subjects. J Hypertens. 1997;15(8):839–44.
Buchholz K, et al. Enhanced affective startle modulation in salt-sensitive subjects. Hypertension. 2001;38(6):1325–9.
Schneider MP, et al. Impaired sodium excretion during mental stress in mild essential hypertension. Hypertension. 2001;37(3):923–7.
Light KC, Turner JR. Stress-induced changes in the rate of sodium excretion in healthy black and white men. J Psychosom Res. 1992;36(5):497–508.
Notterman DA, Mitchell C. Epigenetics and understanding the impact of social determinants of health. Pediatr Clin North Am. 2015;62(5):1227–40.
Lloyd-Jones DM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5).
Booth JN, et al. Racial differences in maintaining optimal health behaviors into middle age. Am J Prev Med. 2019;56(3):368–75.
Svetkey LP, DASH-Sodium Collaborative Research Group, et al. The DASH diet, sodium intake and blood pressure trial (DASH-sodium): rationale and design. J Am Diet Assoc. 1999;99(8 Suppl):S96-104.
Moore TJ, et al. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. 2001.
Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133(9):2879–82.
Jackson SE, et al. Ethnic differences in magnesium intake in U.S. older adults: findings from NHANES 2005–2016. Nutrients. 2018;10(12).
• McCullough ML, et al. Association of socioeconomic and geographic factors with diet quality in US adults. JAMA Netw Open. 2022;5(6):e2216406. This cross-sectional analysis found that income, educational attainment, and location of residence influenced poor diet quality in a diverse U.S. cohort.
Meyerovitz CV, et al. Social determinants, blood pressure control, and racial inequities in childbearing age women with hypertension, 2001 to 2018. J Am Heart Assoc. 2023;12(5).
Poorolajal J, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS ONE. 2017;12(4):e0174967.
Zhang X, et al. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68(2):324–33.
Cormick G, et al. Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst Rev. 2022;1(1):Cd010037.
Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens (Greenwich). 2008;10(7 Suppl 2):3–11.
Patki PS, et al. Efficacy of potassium and magnesium in essential hypertension: a double-blind, placebo controlled, crossover study. BMJ. 1990;301(6751):521–3.
Huang L, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020:m315.
Stookey JD, et al. Underhydration is associated with obesity, chronic diseases, and death within 3 to 6 years in the U.S. population aged 51–70 years. Nutrients. 2020;12(4):905.
Lartey D, et al. Estimating differences in risk of chronic kidney disease based on water intake in a national sample. Ann Nutr Metab. 2021;77(Suppl. 4):30–2.
Brooks CJ, et al. Racial/ethnic and socioeconomic disparities in hydration status among US adults and the role of tap water and other beverage intake. Am J Public Health. 2017;107(9):1387–94.
Rosinger A, Herrick K. Daily water intake among U.S. men and women, 2009–2012. NCHS Data Brief. 2016;242:1–8.
Robinson AT, et al. Cross-sectional analysis of racial differences in hydration and neighborhood deprivation in young adults. Am J Clin Nutr. 2023.
Roussel R, et al. Plasma copeptin and decline in renal function in a cohort from the community: the prospective D.E.S.I.R. study. Am J Nephrol. 2015;42(2):107–14.
Tasevska I, et al. Copeptin predicts coronary artery disease cardiovascular and total mortality. Heart. 2016;102(2):127–32.
Kanbay M, et al. Acute effects of salt on blood pressure are mediated by serum osmolality. J Clin Hypertens (Greenwich). 2018;20(10):1447–54.
Pescatello LS, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2019;51(6):1314–23.
Mandsager K, et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6).
Li K, Wen M. Racial and ethnic disparities in leisure-time physical activity in California: patterns and mechanisms. Race Soc Probl. 2013;5(3):147–56.
Hawes AM, et al. Disentangling race, poverty, and place in disparities in physical activity. Int J Environ Res Public Health. 2019;16(7).
Pandey A, et al. Determinants of racial/ethnic differences in cardiorespiratory fitness (from the Dallas Heart Study). Am J Cardiol. 2016;118(4):499–503.
Guers JJ, et al. Voluntary wheel running attenuates salt-induced vascular stiffness independent of blood pressure. Am J Hypertens. 2019;32(12):1162–9.
de Souza JA, et al. Swimming training improves cardiovascular autonomic dysfunctions and prevents renal damage in rats fed a high-sodium diet from weaning. Exp Physiol. 2021;106(2):412–26.
Rebholz CM, et al. Physical activity reduces salt sensitivity of blood pressure: the genetic epidemiology network of salt sensitivity study. Am J Epidemiol. 2012;176(Suppl 7):S106–13.
Ahn S, et al. A scoping review of racial/ethnic disparities in sleep. Sleep Med. 2021;81:169–79.
Culver MN, et al. Sleep duration irregularity is associated with elevated blood pressure in young adults. Chronobiol Int. 2022:1–9.
Rogers EM, Banks NF, Jenkins NDM. The effects of sleep disruption on metabolism, hunger, and satiety, and the influence of psychosocial stress and exercise: a narrative review. Diabetes Metab Res Rev. 2023:e3667.
Stock AA, et al. Effects of sleep extension on sleep duration, sleepiness, and blood pressure in college students. Sleep Health. 2020;6(1):32–9.
Acknowledgements
We made our figures using canva.com and biorender.com. We have a biorender license and obtained permissions for the figures.
Funding
This work was supported by National Institutes of Health (NIH) grants K01HL147998 and R15HL165325 (to ATR), UL1TR003096 (pilot funding to ATR and TL-1 Fellowship to SJ).
Author information
Authors and Affiliations
Contributions
SJ and ATR wrote the main manuscript. SJ and ATR prepared the manuscript figures and the table. SJ, SDH, MDC, GJG, and ATR all edited the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeong, S., Hunter, S.D., Cook, M.D. et al. Salty Subjects: Unpacking Racial Differences in Salt-Sensitive Hypertension. Curr Hypertens Rep 26, 43–58 (2024). https://doi.org/10.1007/s11906-023-01275-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-023-01275-z