Abstract
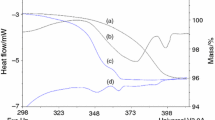
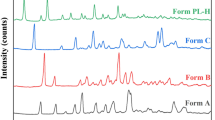
Clindamycin phosphate (CP), an antibacterial agent, has been reported to form several solid-state forms. The crystal structures of two CP solvates, a dimethyl sulfoxide (DMSO) solvate and a methanol/water solvate (solvate V), have been determined by single crystal X-ray diffraction. The properties and transformations of these forms were characterized by powder X-ray diffraction, Single-crystal X-ray diffraction, differential scanning calorimetry, thermo gravimetric analysis, hot-stage microscopy, and dynamic vapor sorption. Very different hydrogen bonding networks exist among the host-host and host-solvent molecules in the two crystal structures, resulting in different moisture stabilities. The thermal stabilities of the two solvates upon heating and desolvation were also studied. When the temperature was above the boiling point of methanol, solvate V converted to a polymorphic phase after a one step desolvation process, whereas the desolvation temperature of the DMSO solvate was below the boiling point of DMSO. At the relative humidity above 43%, the DMSO solvate transformed to a hydrate at 25 °C. In contrast, solvate V did not transform at any of the humidities studied.

Similar content being viewed by others
References
Fujii K, Aoki M, Uekusa H. Solid-state hydration/dehydration of erythromycin A investigated by ab initio powder X-ray diffraction analysis: Stoichiometric and nonstoichiometric dehydrated hydrate. Crystal Growth & Design, 2013, 13(5): 2060–2066
Cui P, Yin Q, Guo Y, Gong J. Polymorphic crystallization and transformation of candesartan cilexetil. Industrial & Engineering Chemistry Research, 2012, 51(39): 12910–12916
Fujii K, Uekusa H, Itoda N, Yonemochi E, Terada K. Mechanism of dehydration-hydration processes of lisinopril dihydrate investigated by ab initio powder X-ray diffraction analysis. Crystal Growth & Design, 2012, 12(12): 6165–6172
Gillon A L, Davey R J, Storey R, Feeder N, Nichols G, Dent G, Apperley D C. Solid state dehydration processes: Mechanism of water loss from crystalline inosine dihydrate. Journal of Physical Chemistry B, 2005, 109(11): 5341–5347
Chakravarty P, Suryanarayanan R. Characterization and structure analysis of thiamine hydrochloride methanol solvate. Crystal Growth & Design, 2010, 10(10): 4414–4420
Renou L, Coste S, Cartigny Y, Petit M, Vincent C, Schneider J M, Coquerel G. Mechanism of hydration and dehydration of ciclopirox ethanolamine (1:1). Crystal Growth & Design, 2009, 9(9): 3918–3927
Jing D, Wang Y, Chen Z, Zhou L, Wang J. Polymorphism and crystal transformation of penicillin sulfoxide. Frontiers of Chemical Science and Engineering, 2011, 5(4): 442–447
Rohani S. Applications of the crystallization process in the pharmaceutical industry. Frontiers of Chemical Engineering in China, 2010, 4(1): 2–9
Lu J, Li Z, Jiang X. Polymorphism of pharmaceutical molecules: Perspectives on nucleation. Frontiers of Chemical Engineering in China, 2010, 4(1): 37–44
Zhang X, Yin Q, Du W, Gong J, Bao Y, Zhang M, Hou B, Hao H. Phase transformation between anhydrate and monohydrate of sodium dehydroacetate. Industrial & Engineering Chemistry Research, 2015, 54(13): 3438–3444
Jali B R, Baruah J B. Polymorphs and solvates of 2-(1,4-dihydro-1,4-dioxonaphthalen-3-ylthio)benzoic acid. Crystal Growth & Design, 2012, 12(6): 3114–3122
Zimmermann A, Frøstrup B, Bond A D. Polymorphs of pridopidine hydrochloride. Crystal Growth & Design, 2012, 12(6): 2961–2968
Shevchenko A, Belle D D, Tiittanen S, Karjalainen A, Tolvanen A, Tanninen V P, Haarala J, Mäkelä M, Yliruusi J, Miroshnyk I. Coupling polymorphism/Solvatomorphism and physical stability evaluation with early salt synthesis optimization of an investigational drug. Organic Process Research & Development, 2011, 15(3): 666–672
Aitipamula S, Chow P S, Tan R B H. Solvates and polymorphic phase transformations of 2-chloro-4-nitrobenzoic acid. CrystEng-Comm, 2011, 13(3): 1037–1045
Braun D E, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser U J. Stability of solvates and packing systematics of nine crystal forms of the antipsychotic drug aripiprazole. Crystal Growth & Design, 2009, 9(2): 1054–1065
Bisht K K, Kathalikkattil A C, Suresh E. Hydrogen-bonded one-and two-dimensional hybrid water-chloride motifs. Crystal Growth & Design, 2012, 12(2): 556–561
Jungwirth P, Tobias D J. Ions at the air/water interface. Journal of Physical Chemistry B, 2002, 106(25): 6361–6373
Hossain M A, Saeed M A, Fronczek F R, Wong B M, Dey K R, Mendy J S, Gibson D. Charge-assisted encapsulation of two chlorides by a hexaprotonated azamacrocycle. Crystal Growth & Design, 2010, 10(4): 1478–1481
Vippagunta S R, Brittain H G, Grant D J W. Crystalline solids. Advanced Drug Delivery Reviews, 2001, 48(1): 3–26
Bakhoda A, Khavasi H R, Safari N. Discrete cubane-like bromidewater cluster. Crystal Growth & Design, 2011, 11(4): 933–935
Matsuo K, Matsuoka M. Solid-state polymorphic transition of theophylline anhydrate and humidity effect. Crystal Growth & Design, 2007, 7(2): 411–415
Singh D, Baruah J B. Structural study on solvates of dopaminebased cyclic imide derivatives. Crystal Growth & Design, 2011, 11(3): 768–777
Morris K R, Griesser U J, Eckhardt C J, Stowell J G. Theoretical approaches to physical transformations of active pharmaceutical ingredients during manufacturing processes. Advanced Drug Delivery Reviews, 2001, 48(1): 91–114
Babu N J, Nangia A. Multiple Z′ in carboxylic acid-pyridine trimer synthon and Kagomé lattice in the structure of 5-methylpyrazine-2,3-dicarboxylic acid. Crystal Growth & Design, 2006, 6(9): 1995–1999
Bhattacharya S, Sameena J, Saha B K. Solvates of ajmaline and twodimensional isostructurality between methanol and ethanol solvates. Crystal Growth & Design, 2011, 11(4): 905–909
Liu Z, Yin Q, Zhang X, Gong J, Xie C. Characterization and structure analysis of cefodizime sodium solvates crystallized from water and ethanol binary solvent mixtures. Industrial & Engineering Chemistry Research, 2014, 53(8): 3373–3377
Stevens D L, Maier K A, Laine B M, Mitten J E. Comparison of clindamycin, rifampin, tetracycline, metronidazole, and penicillin for efficacy in prevention of experimental gas gangrene due to Clostridium perfringens. Journal of Infectious Diseases, 1987, 155(2): 220–228
Ran Y, Dong W, Wu S, Wang J, Gong J. Transformations among the new solid-state forms of clindamycin phosphate. Organic Process Research & Development, 2013, 17(11): 1445–1450
ASTM. Standard Practice for Maintaining Constant Relative Humidity by Means of Aqueous Glycerin Solutions. ASTM E104-85, 1996
Nyqvist H, Nyqvist H. Saturated salt solutions for maintaining specified relative humidity. Energy, 1980, 10: 1085–1090
Fujii K, Ashida Y, Uekusa H, Hirano S, Toyota S, Toda F, Pan Z, Harris K D M. Vapour induced crystalline transformation investigated by ab initio powder X-ray diffraction analysis. Crystal Growth & Design, 2009, 9(2): 1201–1207
Chavez K J, Guevara M, Rousseau R W. Characterization of solvates formed by sodium naproxen and an homologous series of alcohols. Crystal Growth & Design, 2010, 10(8): 3372–3377
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant Nos. 81361140344 and 2136164), the National High Technology Research and Development Program of China (2015AA021002) and the Major National Scientific Instrument Development Project (No.21537812).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gong, J., Zhang, D., Ran, Y. et al. Solvates and polymorphs of clindamycin phosphate: Structural, thermal stability and moisture stability studies. Front. Chem. Sci. Eng. 11, 220–230 (2017). https://doi.org/10.1007/s11705-017-1624-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-017-1624-4