Abstract
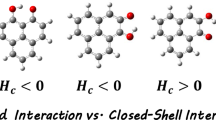
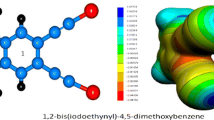
Weak hydrogen bonds (WHBs) have been the focus of intense research in recent years, particularly in structural chemistry and molecular biology. Several studies have shown that WHBs play a significant role in both molecular recognition and supramolecular associations. However, the geometrical, electronic, and energetic properties of WHBs remain incompletely characterized. This work presents a combined structural and theoretical investigation into the crystal arrangement of a 1-chloro-4-methoxybenzene derivative. Our study highlights the significant role of weak hydrogen bonding (C–H···X, where X = O, Cl, π, or C–H) as the primary driving force behind the crystal packing. The geometrical features of these WHBs were analyzed structurally. Computational analysis of several selected dimeric substructures was then performed using dispersion-corrected DFT (wB97X-D/aug-cc-pVTZ), MEP and QTAIM approaches to evaluate their electronic properties and energetics. The results showed that cooperative effects and synergies exist among C–H···O/Cl/π interactions. Moreover, the stabilizing contribution of the weakest but attractive C–H···H–C contacts was also confirmed. Natural bond orbital (NBO) methodology further gave insights into the charge transfer and molecular orbital interactions. The findings from this work may have valuable implications for the understanding, characterization, and practical application of weak hydrogen bonding.








Similar content being viewed by others
References
Abad N, Guelmami L, Haouas A, Hajji M, El Hafi M, Sebhaoui J, Guerfel T, Mague JT, Essassi EM, Ramli Y (2023) Synthesis, non-covalent interactions and chemical reactivity of 1-pentyl-3-phenylquinoxalin-2(1H)-one–structural and computational studies. J Mol Struct 1286:135622. https://doi.org/10.1016/J.MOLSTRUC.2023.135622
Ajitha M, Huang KW (2016) Non-classical C-H···X hydrogen bonding and its role in asymmetric organocatalysis. Synthesis 48(20):3449–3458. https://doi.org/10.1055/s-0035-1562475
Al-Hamdani YS, Tkatchenko A (2019) Understanding non-covalent interactions in larger molecular complexes from first principles. J Chem Phys 150:010901. https://doi.org/10.1063/1.5075487
Ballesteros F, Dunivan S, Lao KU (2021) Coupled cluster benchmarks of large noncovalent complexes: the L7 dataset as well as DNA–ellipticine and buckycatcher–fullerene. J Chem Phys 154:154104. https://doi.org/10.1063/5.0042906
Belkhiria M, Hajji M, Mechria A, Elmgirhi SMH, Habib MA, Cruz TFC, Gomes CSB, Gomes PT, Bel-Hadj-Tahar R, Guerfel T, Msaddek M (2020) Neutral nickel(II) complex bearing hemilabile N, S-donor ligands–structural, Hirshfeld surfaces and DFT studies. Mol Cryst Liq Cryst 709:98–110. https://doi.org/10.1080/15421406.2020.1829311
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Bulusu G, Desiraju GR (2020) Strong and weak hydrogen bonds in protein-ligand recognition. J Indian Inst Sci 100(1):31–41. https://doi.org/10.1007/s41745-019-00141-9
Calhorda MJ (2000) Weak hydrogen bonds: theoretical studies. Chem Commun 10:801–809. https://doi.org/10.1039/a900221i
Czernek J, Brus J, Czerneková V (2022) A cost effective scheme for the highly accurate description of intermolecular binding in large complexes. Int J Mol Sci 23:15773. https://doi.org/10.3390/ijms232415773
Danovich D, Shaik S, Neese F, Echeverría J, Aullón G, Alvarez S (2013) Understanding the nature of the CH···HC interactions in alkanes. J Chem Theory Comput 9:1977–1991. https://doi.org/10.1021/ct400070j
Dennington R, Keith TA, Millam JM (2016) Gaussview version 6. Semichem Inc., Shawnee Mission KS
Derewenda ZS (2023) C-H groups as donors in hydrogen bonds: a historical overview and occurrence in proteins and nucleic acids. Int J Mol Sci 24(17):13165. https://doi.org/10.3390/ijms241713165
Dhifaoui S, Hajji M, Nasri S, Guerfel T, Daran J-C, Nasri H (2018) A new high-spin iron(III) bis(aqua) complex with the meso-tetra(para-chlorophenyl)porphyrin: X-ray crystallography, Hirshfeld surface analysis, magnetic, EPR and electrochemical properties. Res Chem Intermed 44:7259–7276. https://doi.org/10.1007/s11164-018-3555-1
Dragelj JLj, Janjić GV, Veljković DŽ, Zarić SD, (2013) Crystallographic and ab initio study of pyridine CH–O interactions: linearity of the interactions and influence of pyridine classical hydrogen bonds. CrystEngComm 15(48):10481. https://doi.org/10.1039/c3ce40759d
Dutta J, Sahu AK, Bhadauria AS, Biswal HS (2022) Carbon-Centered hydrogen bonds in proteins. J Chem Inf Model 62:1998–2008. https://doi.org/10.1021/acs.jcim.2c00015
Echeverría J, Aullón G, Alvarez S (2017) Intermolecular interactions in group 14 hydrides: Beyond C-H···H-C contacts. Int J Quantum Chem 117:1–15. https://doi.org/10.1002/qua.25432
Famulari A, Martí-Rujas J (2022) Host-guest chemistry of M12L8 Poly-[n]-catenanes: inclusion process by switchable “closed–open” dynamic channels. Cryst Growth Des 22:4494–4502. https://doi.org/10.1021/acs.cgd.2c00423
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision D01. Gaussian Inc., Wallingford
Galvez CE, Piro OE, Echeverría GA, Robles NL, Lezama JOG, Sankaran SV, Thamotharan S, Villecco MB, del Loandos M, H, Gil DM, (2022) Experimental and theoretical insights into the formation of weak hydrogen bonds and H⋯H bonding interactions in the solid-state structure of two eucalyptol derivatives. New J Chem 46:5690–5704. https://doi.org/10.1039/D2NJ00428C
Ghosh S, Wategaonkar S (2020) C-H···Y (Y=N, O, π) hydrogen bond: a unique unconventional hydrogen bond. J Indian Inst Sci 100:101–125. https://doi.org/10.1007/s41745-019-00145-5
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Hajji M, Guerfel T (2016) Two new coordination compounds based on polymeric, [CdCl3O] nn−chains or [Cd2.5Cl7O2] n2n− layers:synthesis, crystal structures, physico-chemical properties and theoretical calculations. J Clust Sci 27:1395–1417. https://doi.org/10.1007/s10876-016-1008-9
Hajji M, Abad N, Habib MA, Elmgirhi SMH, Guerfel T (2021a) Computational chemistry methods for modelling non-covalent interactions and chemical reactivity–an overview. J Indian Chem Soc 98:100208. https://doi.org/10.1016/J.JICS.2021.100208
Hajji M, Al-Otaibi JS, Belkhiria M, Dhifaoui S, Habib MA, Elmgirhi SMH, Mtiraoui H, Bel-Hadj-Tahar R, Msaddek M, Guerfel T (2021b) Structural and computational analyses of a 2-propanolammonium-chlorocadmate(II) assembly: pivotal role of hydrogen bonding and H–H interactions. J Mol Struct 1223:128998. https://doi.org/10.1016/j.molstruc.2020.128998
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro:an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17. https://doi.org/10.1186/1758-2946-4-17
Henley A, Bound M, Besley E (2016) Effective binding of methane using a weak hydrogen bond. J Phys Chem A 120(20):3701–3709. https://doi.org/10.1021/acs.jpca.6b03331
Houser J, Kozmon S, Mishra D, Hammerová Z, Wimmerová M, Koča J (2020) The CH–π interaction in protein-carbohydrate binding: bioinformatics and in vitro quantification. Chem - Eur J 26:10769–10780. https://doi.org/10.1002/chem.202000593
Hu X, Vatankhah-Varnoosfaderani M, Zhou J, Li Q, Sheiko SS (2015) Weak hydrogen bonding enables hard, strong, tough, and elastic hydrogels. Adv Mater 27(43):6899–6905. https://doi.org/10.1002/adma.201503724
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Ikeda M, Sah AK, Iwase M, Murashige R, Ishi-i J, Hasegawa M, Kachi-Terajima C, Park K-M, Kuwahara S, Habata Y (2017) C-H⋯Cl − hydrogen bonds in solution and in the solid-state:HgCl2 complexes with cyclen-based cryptands. Dalton Trans 46:3800–3804. https://doi.org/10.1039/C6DT03390C
Itoh Y, Nakashima Y, Tsukamoto S, Kurohara T, Suzuki M, Sakae Y, Oda M, Okamoto Y, Suzuki T (2019) N+-C-H···O Hydrogen bonds in protein-ligand complexes. Sci Rep 9:767. https://doi.org/10.1038/s41598-018-36987-9
Jena S, Dutta J, Tulsiyan KD, Sahu AK, Choudhury SS, Biswal HS (2022) Noncovalent interactions in proteins and nucleic acids:beyond hydrogen bonding and π-stacking. Chem Soc Rev 51:4261–4286. https://doi.org/10.1039/D2CS00133K
Khavasi HR, Balmohammadi Y, Naghavi SS (2019) Phenomenal observation of attractive intermolecular CH⋯HC Interaction in a mercury (II) complex: an experimental and first-principles study. ChemistrySelect 4:10246–10253. https://doi.org/10.1002/slct.201901932
Kiessling LL, Diehl RC (2021) CH−π interactions in glycan recognition. ACS Chem Biol 16:1884–1893. https://doi.org/10.1021/acschembio.1c00413
Liu M, Yin C, Chen P, Zhang M, Parkin S, Zhou P, Li T, Yu F, Long S (2017) sp2 CH⋯Cl hydrogen bond in the conformational polymorphism of 4-chloro-phenylanthranilic acid. CrystEngComm 19:4345–4354. https://doi.org/10.1039/C7CE00772H
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Mahmudov KT, Kopylovich MN, Guedes da Silva MFC, Pombeiro AJL (2017) Non-covalent interactions in the synthesis of coordination compounds: recent advances. Coord Chem Rev 345:54–72. https://doi.org/10.1016/j.ccr.2016.09.002
Matczak P (2016) Intramolecular C-H···H–C contacts in diheteroaryl ketones and thioketones: a theoretical analysis. Bull Chem Soc Jpn 89:92–102. https://doi.org/10.1246/bcsj.20150229
Nasri S, Hajji M, Guergueb M, Dhifaoui S, Marvaud V, Loiseau F, Molton F, Roisnel T, Guerfel T, Nasri H (2021) Spectroscopic, electrochemical, magnetic and structural characterization of an hexamethylenetetramine Co(II) Porphyrin complex–application in the catalytic degradation of vat yellow 1 dye. J Mol Struct 1231:129676. https://doi.org/10.1016/j.molstruc.2020.129676
Novikov AS (2022) Non-covalent interactions in organic, organometallic, and inorganic supramolecular systems relevant for medicine, materials science, and catalysis. Crystals 12:246. https://doi.org/10.3390/cryst12020246
Pal D, Charaya H, Chakraborty S (2023) An experimental exploration of C−H⋅⋅⋅X hydrogen bond in [CHCl 3−X(CH3)2] complexes (X=O, S, and Se). ChemPhysChem 24(15):e202300124. https://doi.org/10.1002/cphc.202300124
Perlstein J (2001) The weak hydrogen bond in structural chemistry and biology. J Am Chem Soc 123(1):191–192. https://doi.org/10.1021/ja0047368
Riu MLY, Bistoni G, Cummins CC (2021) Understanding the nature and properties of hydrogen-hydrogen bonds: the stability of a bulky phosphatetrahedrane as a case study. J Phys Chem A 125:6151–6157. https://doi.org/10.1021/acs.jpca.1c04046
Rösel S, Quanz H, Logemann C, Becker J, Mossou E, Cañadillas-Delgado L, Caldeweyher E, Grimme S, Schreiner PR (2017) London dispersion enables the shortest intermolecular hydrocarbon H···H contact. J Am Chem Soc 139:7428–7431. https://doi.org/10.1021/jacs.7b01879
Sagan F, Filas R, Mitoraj M (2016) Non-Covalent interactions in hydrogen storage materials LiN(CH3)2BH3 and KN(CH3)2BH3. Crystals 6:28. https://doi.org/10.3390/cryst6030028
Sato H (2021) Weak hydrogen bonding in biodegradable polymers. Pp 435–452 in: spectroscopic techniques for polymer characterization. Wiley. https://doi.org/10.1002/9783527830312.ch16
Scheiner S (2006) Contribution of CH· · ·X Hydrogen Bonds to Biomolecular Structure. In: Grabowski, S.J. (eds) Hydrogen Bonding–New Insights. 3:263–292 Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-4853-1_7
Soudani S, Hajji M, Mi JX, Jelsch C, Lefebvre F, Guerfel T, Ben Nasr C (2020) Synthesis, structure and theoretical simulation of a zinc(II) coordination complex with 2,3-pyridinedicarboxylate. J Mol Struct 1199:127015. https://doi.org/10.1016/j.molstruc.2019.127015
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32. https://doi.org/10.1039/B818330A
Spackman PR, Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Jayatilaka D, Spackman MA (2021) CrystalExplorer:a program for hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J Appl Crystallogr 54:1006–1011. https://doi.org/10.1107/S1600576721002910
Steiner T (2003) C-H···O hydrogen bonding in crystals. Crystallogr Rev 9(2–3):177–228. https://doi.org/10.1080/08893110310001621772
Szatylowicz H, Jezierska A, Sadlej-Sosnowska N (2016) Correlations of NBO energies of individual hydrogen bonds in nucleic acid base pairs with some QTAIM parameters. Struct Chem 27:367–376. https://doi.org/10.1007/s11224-015-0724-3
Tzeng SY, Takahashi K, Tzeng WB (2019) Cation spectra of p-chloroanisole and the heavy atom effect on ionization energy. Chem Phys Lett 731:136626. https://doi.org/10.1016/j.cplett.2019.136626
Veljković DŽ, Janjić GV, Zarić SD (2011) Are C-H⋯O interactions linear? The case of aromatic CH donors. CrystEngComm 13(15):5005. https://doi.org/10.1039/c1ce05065f
Wang W, Zhang Y, Liu W (2017) Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog Polym Sci 71:1–25. https://doi.org/10.1016/j.progpolymsci.2017.04.001
Wei X, Abdiaj I, Sambiagio C, Li C, Zysman-Colman E, Alcázar J, Noël T (2019) Visible-light-promoted iron-catalyzed C(sp2)–C(sp3) kumada cross-coupling in flow. Angew Chem Int Ed 58:13030–13034. https://doi.org/10.1002/anie.201906462
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev Phys Chem 35:399–440. https://doi.org/10.1080/0144235X.2016.1192262
Wu R, Chen K, Ma J, Yu Z-X, Zhu S (2020) Synergy of activating substrate and introducing C-H···O interaction to achieve Rh2(II)-catalyzed asymmetric cycloisomerization of 1, n-enynes. Sci China Chem 63:1230–1239. https://doi.org/10.1007/s11426-020-9794-3
Yamauchi O (2016) Noncovalent interactions in biocomplexes. Phys Sci Rev 1(4):20160001. https://doi.org/10.1515/psr-2016-0001
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number “NBU-FFR-2023-0153”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haouas, A. Probing the significance, nature, and energetics of weak hydrogen bonding in the crystal structure of a 1-chloro-4-methoxybenzene derivative through structural and computational modeling. Chem. Pap. 78, 2385–2395 (2024). https://doi.org/10.1007/s11696-023-03245-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03245-w