Abstract
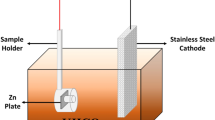
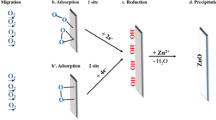
The present article reports synthesis of zinc oxide (ZnO) nanostructures by the single step electrochemical anodization process at room temperature in an aqueous bicarbonate solution. Structural characterizations indicated that the material has grown in the wurtzite phase. Morphological study revealed formation of ZnO nanoflowers with ZnO nanoflakes acting as flower petals having an average width of 53 ± 2 nm and average length of 120 ± 3 nm. ZnO nanoflowers exhibited improved photo electrochemical ability compared to zinc oxide nanowires. The enhanced photo electrochemical ability of nanoflowers could be accounted to the presence of numerous active edge sites in 2D flakes (petals) together with large oxygen vacancy content on the surface of zinc foil revealed by Raman spectroscopy. Additionally, as-fabricated zinc oxide nanoflowers were studied for better light absorption through diffuse reflectance spectroscopy which showed the material possess an optical band gap ~ 3.16 eV. Moreover, Photo electrochemical ability of as fabricated zinc oxide nanoflowers were studied using photo electrochemical analyser and it showed a current density of ~ 60 μA cm− 2. This indicated potential ability of ZnO nanoflowers to serve as UV–visible sunlight-driven photoelectrode materials.
Graphic abstract











Similar content being viewed by others
References
Abdulgafour HI, Hassan Z, Al-Hardan N, Yam FK (2010) Growth of zinc oxide nanoflowers by thermal evaporation method. Phy B Cond Matter 405:2570–2572
Alvi NH, Ali SU, Hussain S, Nur O, Willander M (2011) Fabrication and comparative optical characterization of n-ZnO nanostructures (nanowalls, nanorods, nanoflowers and nanotubes)/p-GaN white-light-emitting diodes. Scri Mater 64:697–700
Anandan S, Ohashi N, Miyauchi M (2010) ZnO-based visible-light photocatalyst Band-gap engineering and multi-electron reduction by co-catalyst. Appl Catalysis B Environ l 100:502–509
Cauda V, Pugliese D, Garino N, Sacco A, Bianco S, Bella F, Gerbaldi C (2014) Multi-functional energy conversion and storage electrodes using flower-like Zinc oxide nanostructures. Energy 65:639–646
Chen PC, Shen G, Zhou C (2008) Chemical sensors and electronic noses based on 1-D metal oxide nanostructures. IEEE Trans Nanotechnol 7:668–682
Cheng AJ, Tzeng Y, Zhou Y, Park M, Wu TH, Shannon C, Lee W (2008) Thermal chemical vapor deposition growth of zinc oxide nanostructures for dye-sensitized solar cell fabrication. ApplPhy Lett 92:092113
Cunha DM, Souza FL (2013a) Facile synthetic route for producing one-dimensional zinc oxide nanoflowers and characterization of their optical properties. J Alloys Compd 577:158–164
Cunha DM, Souza FL (2013b) Facile synthetic route for producing one-dimensional zinc oxide nanoflowers and characterization of their optical properties. J Alloys Compd 577:58–164
Dai Y, Zhang Y, Li QK, Nan CW (2002) Synthesis and optical properties of tetrapod-like zinc oxide nanorods. Chem Phys Lett 358:83–86
Desai MA, Vyas AN, Saratale GD, Sartale SD (2019) Zinc oxide superstructures: recent synthesis approaches and application for hydrogen production via photoelectrochemical water splitting. Inter J Hydrog Energy 44:2091–2127
Fan Z, Lu JG (2005) Zinc oxide nanostructures synthesis and properties. J NanosciNanotechnol 5:1561–1573
Ghosh A, Kumari N, Tewari S, Bhattacharjee A (2013) Structural and optical properties of pure and Al doped ZnO nanocrystals. Ind J Phy 87:1099–1104
Goel S, Sinha N, Yadav H, Godara S, Joseph AJ, Kumar B (2017) Ferroelectric Gd-doped ZnO nanostructures: enhanced dielectric, ferroelectric and piezoelectric properties. Mater Chem Phy 202:56–64
Gomez JL, Tigli O (2013) Zinc oxide nanostructures from growth to application. J Mater Sci 48:612–624
Huang MH, Wu Y, Feick H, Tran N, Weber E, Yang P (2001) Catalytic growth of zinc oxide nanowires by vapor transport. Adv Mater 13:113–116
Huang J, Xia C, Cao L, Zeng X (2008) Facile microwave hydrothermal synthesis of zinc oxide one-dimensional nanostructure with three-dimensional morphology. Mater Sci and Eng B 150:187–193
Huda MN, Yan Y, Wei SH, Al-Jassim MM (2008) Electronic structure of ZnOGaN compounds: asymmetric bandgap engineering. Phyl Rev B 78:195204
Kannan PK, Saraswathi R, Rayappan JBB (2010) A highly sensitive humidity sensor based on DC reactive magnetron sputtered zinc oxide thin film. Sens Actuators A 164:8–14
Krunks M, Dedova T, Açik IO (2006) Spray pyrolysis deposition of zinc oxide nanostructured layers. Thin Solid Films 515:1157–1160
Laurenti M, Garino N, Porro S, Fontana M, Gerbaldi C (2015) Zinc oxide nanostructures by chemical vapour deposition as anodes for Li-ion batteries. J Alloys Compd 640:321–326
Liu M, Nam CY, Black CT, Kamcev J, Zhang L (2013) Enhancing water splitting activity and chemical stability of zinc oxide nanowire photoanodes with ultrathin titania shells. J Phy Chem C 117:13396–13402
Mansournia M, Rafizadeh S, Hosseinpour-Mashkani SM (2015) Hydrothermal synthesis characterization and light harvesting applications of zinc oxide nanostructures. J Mater Sc Mater Elect 26:5839–5846
Mika K, Socha RP, Nyga P, Wiercigroch E, Małek K, Jarosz M, Uchacz T, Sulka GD, Zaraska L (2019) Electrochemical synthesis and characterization of dark nanoporous zinc oxide films. Electrochem Acta 305:349–359
Miles DO, Cameron PJ, Mattia D (2015) Hierarchical 3D ZnO nanowire structures via fast anodization of zinc. J Mater Chem A 3:17569–17577
Momeni MM, Ghayeb Y (2015) Visible light-driven photoelectrochemical water splitting on ZnO–TiO 2 heterogeneous nanotube photoanodes. J Appl Electron 45:557–566
Roy N, Chowdhury A, Paul T, Roy A (2016) Morphological optical and raman characteristics of ZnOnanoflowers on ZnO-seeded Si substrates synthesized by chemical method. J NanosciNanotechnol 16:9738–9745
Sahu K, Kar AK (2019) Morphological, optical, photocatalytic and electrochemical properties of hydrothermally grown ZnOnanoflowers with variation in hydrothermal temperature. Mater Sci Semi Process 104:104648
Suriani AB, Safitri RN, Mohamed A, Alfarisa S, Isa IM, Kamari A, Rusop M (2015) Enhanced field electron emission of flower-like zinc oxide on zinc oxide nanorods grown on carbon nanotubes. Mater Lett 149:66–69
Tien LC, Pearton SJ, Norton DP, Ren F (2008) Synthesis and microstructure of vertically aligned ZnO nanowires grown by high-pressure-assisted pulsed-laser deposition. J Mater Sci 43:6925–6932
Wahab R, Ansari SG, Kim YS, Seo HK, Kim GS, Khang G, Shin HS (2007) Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater Rese Bull 42:1640–1648
Wahyuono RA, Schmidt C, Dellith A, Dellith J, Schulz M, Seyring M, Dietzek B (2016) ZnOnanoflowers-based photoanodes aqueous chemical synthesis, microstructure and optical properties. Open Chem 14:158–169
Wang ZL (2008) Splendid one-dimensional nanostructures of zinc oxide: a new nanomaterial family for nanotechnology. ACS Nano 2:1987–1992
Wang Y, Li X, Wang N, Quan X, Chen Y (2008) Controllable synthesis of ZnOnanoflowers and their morphology-dependent photocatalytic activities. Sep Purif Technol 62:727–732
Wang Y, Liu T, Huang Q, Wu C, Shan D (2016) Synthesis and their photocatalytic properties of Ni-doped ZnO hollow microspheres. J Mater Res 31:2317–2328
Wang BS, Li RY, Zhang ZY, Wu XL, Cheng GA, Zheng RT (2019) An overlapping ZnO nanowire photoanode for photoelectrochemical water splitting. Catal Today 321:100–106
Xiao J, Zhang G, Bai X, Yu L, Zhao X, Guo D (2008) Field emission from zinc oxide nanostructures and its degradation. Vacuum 83:265–272
Zhai YJ, Li JH, Fang X, Chen XY, Fang F, Chu XY, Wang XH (2014) Preparation of cadmium-doped zinc oxide nanoflowers with enhanced photocatalytic activity. Mater Sci Semicond Process 26:225–230
Acknowledgements
The authors acknowledge Prof. MSR Rao, IIT Madras and Prof. S.C Roy, IIT Madras for providing the necessary experimental equipment’s and current density measurement facilities. We are also thankful to National Institute of Technology Srinagar J&K and MHRD for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tantray, A.M., Shah, M.A. Photo electrochemical stability response of ZnO nanoflowers fabricated through single step electrochemical anodization. Chem. Pap. 75, 1739–1747 (2021). https://doi.org/10.1007/s11696-020-01419-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01419-4