Abstract
Purpose
μ-receptor opioids are associated with unwanted gastrointestinal side effects and respiratory depression. A long-acting non-μ-receptor parenteral opioid is not currently available for management of acute and chronic postsurgical pain (CPSP). This double-blind clinical trial tested an extended-release κ-receptor agonist, sebacoyl dinalbuphine ester (SDE, Naldebain®) for management of surgical pain after laparoscopic bariatric surgery.
Materials and Methods
Patients were randomly assigned to receive a single intramuscular injection of SDE (150 mg, n = 30) or vehicle solution (n = 30) at > 12 h before surgery. All patients received standard perioperative multimodal analgesia (MMA). The primary endpoint was the pain intensity in the beginning 7 days after operation. The secondary endpoints were adverse reactions up to 7 days and incidence of CPSP at 3 months after surgery.
Results
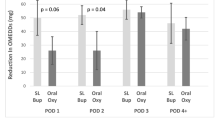
Compared with placebos, the area under curves of visual analog scale (VAS) for 0–48 h after operation were significantly reduced in SDE group (143.3 ± 65.4 and 105.9 ± 36.3, P = 0.025). There were significantly fewer patients in the SDE group who had moderate-to-severe pain (VAS ≥ 4) (16.7% vs 50%; P = 0.012) at postoperative 48 h. Pain intensities were similar between the two groups at 72 h and 7 days postoperatively. The incidence of CPSP at 3 months was not different. SDE did not increase drug-related systemic adverse events.
Conclusion
In addition to the standard perioperative MMA, a single-dose injection of long-acting κ-receptor agonist SDE provides significantly better pain management for 48 h following laparoscopic bariatric surgery. A long-acting κ-receptor agonist opioid could improve in-hospital pain management and potentiate early discharge after operation without increasing drug-related systemic complications.
Graphical Abstract




Similar content being viewed by others
Data Availability
Data and associated documentation will be available to users from the corresponding authors on reasonable request.
Abbreviations
- ASA PS:
-
American Society for Anesthesiologist physical statuses
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CPSP:
-
Chronic postsurgical pain
- ERAS:
-
Enhanced recovery after surgery
- IBW:
-
Ideal body weight
- MMA:
-
Multimodal analgesia
- MME:
-
Morphine milligram equivalent
- NRS:
-
Numeric rating scale
- NSAID:
-
Non-steroidal anti-inflammatory drugs
- PACU:
-
Post-anesthesia care unit
- PONV:
-
Postoperative nausea and vomiting
- QoL:
-
Quality of life
- SDE:
-
Sebacoyl dinalbuphine ester
- SF-12:
-
12-Item short form
- VAS:
-
Visual analog scale
References
Huang YM, Lin YK, Lee WJ, et al. Long-term outcomes of metabolic surgery in overweight and obese patients with type 2 diabetes in Asia. Diabetes Obes Metab. 2021;23(3):742–53.
El-Sherbiny W, Saber W, Askalany AN, et al. Effect of intra-abdominal instillation of lidocaine during minor laparoscopic procedures. Int J Gynaecol Obstet. 2009;106(3):213–5.
Golzari SE, Nader ND, Mahmoodpoor A. Underlying mechanisms of postoperative pain after laparoscopic surgery. JAMA Surg. 2016;151(3):295–6.
Gribsholt SB, Pedersen AM, Svensson E, et al. Prevalence of self-reported symptoms after gastric bypass surgery for obesity. JAMA Surg. 2016;151(6):504–11.
Nasser K, Verhoeff K, Mocanu V, et al. New persistent opioid use after bariatric surgery: a systematic review and pooled proportion meta-analysis. Surg Endosc. 2023;37(1):703–714. https://doi.org/10.1007/s00464-022-09291-x.
Blichfeldt-Eckhardt MR, Ording H, Andersen C, et al. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155(11):2400–7.
Machelska H, Celik M. Advances in achieving opioid analgesia without side effects. Front Pharmacol. 2018;9:1388.
Huang CC, Sun WZ, Wong CS. Prevention of chronic postsurgical pain: the effect of preventive and multimodal analgesia. Asian J Anesthesiol. 2018;56(3):74–82.
Yeh YC, Lin TF, Lin FS, et al. Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain. Br J Anaesth. 2008;101(4):542–8.
Li CJ, Ku MY, Lu CY, et al. In vitro and in vivo release of dinalbuphine sebacate extended release formulation: effect of the oil ratio on drug release. Int J Pharm. 2017;531(1):306–12.
Tien YE, Huang WC, Kuo HY, et al. Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after intramuscular injection of dinalbuphine sebacate in an extended-release formulation. Biopharm Drug Dispos. 2017;38(8):494–7.
Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7.
Ristanto A, Caltabiano ML. Psychological support and well-being in post-bariatric surgery patients. Obes Surg. 2019;29(2):739–43.
Horsley RD, Vogels ED, McField DAP, et al. Multimodal postoperative pain control is effective and reduces opioid use after laparoscopic roux-en-y gastric bypass. Obes Surg. 2019;29(2):394–400.
Stenberg E, Dos Reis Falcão LF, O’Kane M, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: a 2021 update. World J Surg. 2022;46(4):729–51.
Hung KC, Chiu CC, Hsu CW, et al. Impact of opioid-free anesthesia on analgesia and recovery following bariatric surgery: a meta-analysis of randomized controlled studies. Obes Surg. 2022;32(9):3113–24.
Lin A, Verhoeff K, Mocanu V, et al. Opioid prescribing practices following bariatric surgery: a systematic review and pooled proportion meta-analysis. Surg Endosc. 2023;37(1):62–74. https://doi.org/10.1007/s00464-022-09481-7.
Tan WH, Ford J, Kindel T, et al. Implementation of a standardized multimodal pain regimen significantly reduces postoperative inpatient opioid utilization in patients undergoing bariatric surgery. Surg Endosc. 2022. https://doi.org/10.1007/s00464-022-09482-6.
Lloret-Linares C, Lopes A, Declèves X, et al. Challenges in the optimisation of post-operative pain management with opioids in obese patients: a literature review. Obes Surg. 2013;23(9):1458–75.
Camilleri M, Lembo A, Katzka DA. Opioids in gastroenterology: treating adverse effects and creating therapeutic benefits. Clin Gastroenterol Hepatol. 2017;15(9):1338–49.
Jaillon P, Gardin ME, Lecocq B, et al. Pharmacokinetics of nalbuphine in infants, young healthy volunteers, and elderly patients. Clin Pharmacol Ther. 1989;46(2):226–33.
Zeng Z, Lu J, Shu C, et al. A comparision of nalbuphine with morphine for analgesic effects and safety: meta-analysis of randomized controlled trials. Sci Rep. 2015;5:10927.
Aitkenhead AR, Lin ES, Achola KJ. The pharmacokinetics of oral and intravenous nalbuphine in healthy volunteers. Br J Clin Pharmacol. 1988;25(2):264–8.
Yeh CY, Jao SW, Chen JS, et al. Sebacoyl dinalbuphine ester extended-release injection for long-acting analgesia: a multicenter, randomized, double-blind, and placebo-controlled study in hemorrhoidectomy patients. Clin J Pain. 2017;33(5):429–34.
Chang TK, Huang CW, Su WC, et al. Extended-release dinalbuphine sebacate versus intravenous patient-controlled analgesia with fentanyl for postoperative moderate-to-severe pain: a randomized controlled trial. Pain Ther. 2020;9(2):671–81.
Gribsholt SB, Svensson E, Richelsen B, et al. Rate of acute hospital admissions before and after roux-en-y gastric bypass surgery: a population-based cohort study. Ann Surg. 2018;267(2):319–25.
Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291–301.
Dinges HC, Otto S, Stay DK, et al. Side effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Analg. 2019;129(4):1153–62.
Lee SO, Huang LP, Wong CS. Preoperative administration of extended-release dinalbuphine sebacate compares with morphine for post-laparoscopic cholecystectomy pain management: a randomized study. J Pain Res. 2020;13:2247–53.
Chang SH, Chang TC, Chen MY, et al. Comparison of the efficacy and safety of dinalbuphine sebacate, patient-controlled analgesia, and conventional analgesia after laparotomy for gynecologic cancers: a retrospective study. J Pain Res. 2021;14:1763–71.
Funding
This study was funded in part by the Ministry of Science and Technology of Taiwan (grant number MOST 109–2314-B-650–007-MY2) and institutional grants from E-Da Hospital, located in Taiwan (EDPJ109034 and EDPJ109064 to CFL).
Author information
Authors and Affiliations
Contributions
Ying-En Lee, MD, helped with conceptualization of the study and study design, study drug administration, data collection, patient follow-up, statistical analysis, interpretation of data, and drafting the manuscript. The author approved the final version of manuscript.
Shao-Ye Wang, RN, helped with patient administration, assistance in drug administration, clinical data collection, and patient follow-up. The author approved the final version of manuscript.
Jian-Han Chen, MD, helped with conceptualization of the study and study design and performed surgery, patient follow-up, statistical analysis, and drafting of the manuscript. The author approved the final version of manuscript.
Chung-Yen Chen, MD., helped with study design, performed surgery, patient follow-up, and drafting of the manuscript. The author approved the final version of manuscript.
Yow-Ling Shiue, PhD., helped with conceptualization of the study and study design and interpretation of data. The author approved the final version of manuscript.
Tien-Chou Soong, MD., helped with study design; performed surgery, patient follow-up, supervision of study progress, statistical analysis, and interpretation of data; and critically revised the manuscript. The author approved the final version of manuscript.
Chen-Fuh Lam, MD, PhD., helped with the study design, supervision of study progress, statistical analysis, and interpretation of data, obtained research funds, critically revised the manuscript, and is responsible for the submission of the manuscript. The author approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All procedures involving human participants in this study were conducted in accordance with the institutional ethical standards and with the 1964 Helsinki declaration and its later amendments.
Conflict of Interest
This was a PI-initiated clinical research project, and the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Long-acting non-μ-receptor opioids were previously not available for postsurgical pain management.
• Injection of a long-acting κ-receptor agonist sebacoyl dinalbuphine ester (SDE) significantly improves surgical pain intensity up to 48 h following laparoscopic bariatric surgery.
• SDE does not increase systemic adverse events but may induce focal reactions in patients with obesity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, YE., Wang, SY., Chen, JH. et al. Efficacy and Safety of Parenteral Injection of an Extended Release κ-receptor Opioid Sebacoyl Dinalbuphine Ester for Acute and Chronic Pain After Laparoscopic Bariatric Surgery: a Randomized, Placebo-Controlled, Double-Blind Trial. OBES SURG 33, 1192–1201 (2023). https://doi.org/10.1007/s11695-023-06502-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06502-9