Abstract
Objective
In patients undergoing laparoscopic radical gastrectomy, the use of subcostal transversus abdominis plane block (STAPB) for completely opioid-free postoperative pain management lacks convincing clinical evidence.
Methods
This study included 112 patients who underwent laparoscopic radical gastrectomy at the 900TH Hospital of the Joint Logistics Support Force from October 2020 to March 2022. Patients were randomly divided into (1:1) continuous opioid-free STAPB (C-STAPB) group and conventional group. In the C-STAPB group, 0.2% ropivacaine (bilateral, 20 ml per side) was injected intermittently every 12 h through a catheter placed on the transverse abdominis plane for postoperative pain management. The conventional group was treated with a conventional intravenous opioid pump (2.5 μg/kg sufentanil and 10 mg tropisetron, diluted to 100 ml with 0.9% NS). The primary outcomes were the accumulative area under the curve of the numeric rating scale (NRS) score at 24 and 48 h postoperatively at rest and during movement. The secondary outcomes were postoperative recovery outcomes, postoperative daily food intake, and postoperative complications.
Results
After exclusion (n = 16), a total of 96 patients (C-STAPB group, n = 46; conventional group, n = 49) were included. We found there were no significant differences in the cumulative AUC of NRS score PACU-24 h and PACU-48 h between the C-STAPB group and conventional group at rest [(mean difference, 1.38; 95% CI, − 2.21 to 4.98, P = 0.447), (mean difference, 1.22; 95% CI, − 6.20 to 8.65, P = 0.744)] and at movement [(mean difference, 2.90; 95% CI, − 3.65 to 9.46; P = 0.382), (mean difference, 4.32; 95% CI, − 4.46 to 13.1; P = 0.331)]. The 95% CI upper bound of the difference between rest and movement in the C-STAPB group was less than the inferior margin value (9.5 and 14 points), indicating the non-inferiority of the analgesic effect of C-STPAB. The C-STAPB group had faster postoperative recovery profiles including earlier bowel movement, defecation, more volume of food intake postoperative, and lower postoperative nausea and vomiting compared to conventional groups (P < 0.001).
Conclusions
After laparoscopic radical gastrectomy, the analgesic effect of C-STAPBP is not inferior to the traditional opioid-based pain management model.
Trial registration
ChiCTR2100051784.
Similar content being viewed by others
Introduction
Gastric cancer is an important global disease, mainly distributed in East Asia, Eastern Europe, and South America [1]. It is estimated that there are more than 1 million new cases of gastric cancer each year, which is the fifth largest malignant tumor in the world [1]. At present, laparoscopic surgery has become the mainstream of gastric cancer surgery. Although laparoscopic surgery has significantly reduced the patient’s physical trauma, there are still 5–10-cm surgical incisions, and postoperative pain is still serious [2]. Opioids are still the cornerstone of anesthesia and postoperative pain management in laparoscopic radical gastrectomy. However, because opioid receptors are widely present in various organs of the human body, they can also cause opioid-related adverse events (ORAE), such as nausea, vomiting, dizziness, intestinal paralysis, and respiratory depression while exerting analgesic effects [3].
Related studies have shown that the application of a large number of opioids during the perioperative period would significantly prolong the length of hospital stay [4]. According to statistics, the average incidence of ORAE was as high as about 20%, and the incidence of ORAE after gastrointestinal surgery was higher [4]. Therefore, it is important to minimize the use of opioids after gastrointestinal surgery. The postoperative analgesia model opioid-free is ideal, but it still needs to be studied.
Transversus abdominis plane block (TAPB) is the most common regional anesthesia used to manage postoperative pain after abdominal surgery [5,6,7]. However, this technique provides a limited duration of postoperative analgesia (last 8–12 h) [8]. Compared with single injection STAPB, continuous transversus abdominis plane block (C-STAPB) can reduce postoperative pain and opioid dosage [8]. In various abdominal surgeries, C-STAPB can be used as a feasible alternative to subdural catheter block and is a safe and effective analgesic method [9]. At present, there is no relevant study to compare the analgesic effect of patient-controlled intravenous analgesia pump (PCIA) and C-STAPB after laparoscopic radical gastrectomy. Most importantly, as far as we know, whether C-STAPB can achieve complete opioid-free postoperative pain management in patients undergoing laparoscopic radical gastrectomy remains to be studied.
Therefore, we designed a C-STAPB procedure to achieve complete opioid-free postoperative pain management. In this randomized controlled trial, bilateral STABP was performed simultaneously with continuous intermittent injection of a long-half-life local anesthetic (ropivacaine). The purpose of this study was to evaluate the non-inferiority of opioid-free C-STAPB in pain management after laparoscopic radical gastrectomy compared with traditional opioid-based regimens.
Materials and methods
Trial design
This open-label, randomized, controlled, non-inferiority trial was approved by the 900TH Hospital of the Joint Logistics Support Force Institutional Review Board (2,014,046) and conducted in accordance with the Helsinki Declaration. This study follows the comprehensive standard guidelines for test reports. Written informed consent of all patients was obtained. This study was registered in ChiCTR.org.cn (ChiCTR2100051784).
Participants
This study was conducted from October 2020 to March 2022 at the 900TH Hospital of the Joint Logistics Support Force, one of the largest cancer centers in southeastern China. The sample size was estimated using PASS software, because there were not enough relevant references in the literature at the time. As advised, the noninferiority was established at 1/5 of the conventional group mean [10, 11]. The cumulative AUCs of PACU-24 h and PACU-48 h at the movement of the ten attempts of conventional procedures were 48.20 ± 16.20 and 86.40 ± 42.26. Based on this, we determined that the non-inferiority values of the average cumulative AUC of PACU-24 h and PACU-48 h at movement were 9.5 and 14 points. Based on that, a total of 100 patients were calculated (1:1 ratio) to be needed (50 in each group) for the primary analysis at rest, with a significance level of one side α = 0.025, a power of 1 − β = 0.85, and 25% contingency factor. Similarly, a total of 60 patients were calculated (1:1 ratio) to be needed (30 in each group) for the primary analysis at movement.
Inclusion criteria are as follows: (1) the diagnosis of the gastric malignant tumor was confirmed by CT, MRI, gastroscopy, and pathological diagnosis; (2) preoperative evaluation of lymph node metastasis without organ and distant metastasis and in line with laparoscopic surgery; (3) American Society of Anesthesiologists (ASA) grade I/III; (4) age 18–75 years old.
Exclusion criteria are as follows: (1) unable to cooperate with the experiment due to language communication disorders or severe cognitive impairment; (2) allergic to drug addicts or experimental-related drugs; (3) other surgical history.
Randomization and masking
Patients were randomly divided into the C-STAPB group or conventional group at a ratio of 1:1. Each patient was randomly grouped by a third-party professional medical staff through a computer-generated random number. The odd random number was the conventional group, and the even random number was the C-STAPB group. For ethical reasons, patients and doctors who took care of the patients since intraoperatively and postoperatively did not blind the patient allocation group.
Interventions
In the C-STAPB group, after the abdominal incision was closed, the transverse abdominal plane block catheter was established on the lateral side of the bilateral meniscus of the surgical incision. Using ultrasonic positioning, the trocar was inserted from the tail end to the head end into the gap between the internal oblique muscle and the transverse abdominal muscle. 5 ml 0.9% NS was injected to expand the space, and then the trocar was pushed about 0.5–1.0 cm to ensure stability and reliability. Then, the hard needle was withdrawn, the catheter was fixed locally with a transparent paste, and the length of catheter exposure was recorded. After confirming the appropriate position of the catheter, the first dose was injected (40 mL 0.2% ropivacaine, 20 mL on each side). Ultrasound was used to observe the liquid diffusion in the injection space. Repeat the same procedure on the opposite side. In the C-STAPB group, the drug was administered once every 12 h after surgery through a fixed catheter until 48 h after surgery in the post-anesthesia care unit (PACU) (20 ml per side, 5 postoperative injections). In the conventional group, patients received a conventional opioid PCIA pump (2.5 μg/kg sufentanil and 10 mg tropisetron, diluted to 100 mL with 0.9% NS). Considering the strict bias control, all intravenous pumps were locked to administer only the basic dose (2 mL/h), of which patient-controlled additional use was disabled (Fig. 1).
Trial intervention diagram. A The intervention of the C-STAPB group. B The intervention of the conventional group. (a) Bilateral transverse abdominal muscle plane catheterization. (b) Transversus abdominis plane block under ultrasound (red arrow for local anesthetic, orange arrow for the needle, blue arrow for the external oblique, yellow arrow for the internal oblique, green arrow for the transversus abdominis muscle). Abbreviations: C-STAPB, continuous subcostal transversus abdominis plane block
All patients underwent general anesthesia by the same experienced anesthesiologist and the same anesthesia regimen was used. All surgeries are performed by experienced gastrointestinal surgeons. Postoperative pain numeric rating scale (NRS, score: 0–10, the higher the score, the more severe the pain) was used to assess the pain in the first 4 days after surgery. The decision to provide rescue analgesia was made by the surgeon and was only considered when the patient requests and the NRS ≥ 4. Flurbiprofen (50 mg) was the only non-steroidal anti-inflammatory drug (NSAID) used to rescue analgesia. The C-STAPB catheter and PCIA pump were removed 48 h after the operation.
Outcomes
The primary outcomes
The primary outcomes included the cumulative area under the curve (AUC) of the pain NRS score at 24 and 48 h after surgery both at rest and during movement. Pain score at rest and movement at 24 and 48 h was assessed using the numeric rating scale from 0 to 10, According to the NRS score and postoperative time, the overall AUC of pain score at rest and during movement at 48 h after surgery was compared. The time points were set at PACU (0 h), 24 h, and 48 h after leaving PACU. The calculation formula of AUC is as follows: AUC = ∑ (NRSi + NRSj) * (tj − ti)/2, when NRSi and NRSj were the NRS scores corresponding to two adjacent observation time points tj and ti (j > i), respectively.
The secondary outcomes
The secondary outcomes included the proportion of patients requiring rescue analgesia, postoperative complications, bowel recovery functions, time to first defecation, bowel movement and daily food intake, recovery by time to start ambulation, length of hospital stay, STAPB-related events, and surgical complications. All patients took 40 ml meglumine diatrizoate orally on the second day after the operation, and the time of the radiography agent (meglumine diatrizoate) reaching the ileocecal junction was recorded.
Statistical analysis
All statistical analyses were performed using SPSS 11.0 software. Continuous variables are expressed as mean (SD) or median [interquartile range (IQR)] and compared using Student’s t test or the Mann–Whitney U test, as appropriate. Categorical variables are expressed as absolute frequencies (percentages) and compared using Pearson’s χ2 test or Fisher’s exact test, as appropriate. Student’s t test was used to analyze the NRS scores of the two groups at each time point. Repeated measurement analysis of variance was used for statistical analysis of measurement data that were repeated for the same individual and conformed to the normal distribution. For the primary outcomes, the difference with the 95% confidence interval [CI] is calculated to estimate the non-inferiority. And the non-inferiority values of the average cumulative AUC of PACU-24 h and PACU-48 h were 9.5 and 14 points. P < 0.05 was considered statistically significant.
Results
Basic information of patients
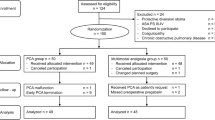
Of the 112 patients screened during the study period, 100 were finally enrolled and randomly assigned to each group (C-STAPB group 49, conventional group 51). In the conventional group, two patients were asked to withdraw from the experiment without reason, and in the C-STAPB group, three patients withdrew from the experiment due to catheter displacement (Fig. 2). The results showed that there was no significant difference in gender, age, body mass index (BMI), preoperative nutritional score, and pathological stage between the two groups (P > 0.05). Although the proportion of laparoscopic radical total gastrectomy in the C-STAPB group was higher than that in the conventional group (58.70% vs 42.86%, P > 0.05), the difference between the two groups was not statistically significant (Table 1).
Cumulative AUC of postoperative NRS
We found no significant differences in the cumulative AUC PACU-24 h and PACU-48 h between the C-STAPB group and conventional group at rest [(mean difference, 1.38; 95% CI, − 2.21 to 4.98, P = 0.447), (mean difference, 1.22; 95% CI, − 6.20 to 8.65, P = 0.744)] and at movement [(mean difference, 2.90; 95% CI, − 3.65 to 9.46; P = 0.382), (mean difference, 4.32; 95% CI, − 4.46 to 13.1; P = 0.331)] (Table 2).
At rest and movement, there was no significant difference in NRS between the C-STAPB group and the conventional group at 0 h, 24 h, and 48 h after surgery (P = 0.80 > 0.05) (P = 0.25 > 0.05). Similarly, there was no significant difference in the rate of patients who required rescue analgesia between the two groups (P > 0.05) at postoperative 24 and 48 h (Table 3).
The secondary outcomes
Postoperative recovery outcomes include time of defecation, bowel movement, time of off-bed, postoperative length of stay, and postoperative daily food intake. The time of defecation (29.56 ± 5.33 vs 50.12 ± 3.90, P = 0.03) and the bowel movement (time of radiography agent to ileocecal junction) (1.76 ± 0.26 vs 2.10 ± 0.39, P = 0.01) in the C-STABP group were significantly earlier than those in the conventional group. In addition, there was a statistically significant difference in postoperative daily food intake between the C-STABP group and the conventional group (P < 0.001) (Table 4).
The incidence of postoperative nausea and vomiting in the C-STABP and conventional groups was significantly different (p = 0.03), but there was no significant difference in other complications between the two groups (Table 4).
Discussion
Additional costs associated with various ORAE and complications increase the medical burden, especially for gastrointestinal surgery [12]. Therefore, such patients urgently need an ideal method to reduce postoperative opioid requirement for pain management. To the best of our knowledge, this is the first study to report that C-STAPB is not inferior to traditional opioids in pain management after laparoscopic radical gastrectomy [13,14,15]. We found that it is feasible to achieve the goal of opioid-free postoperative pain management. In addition, the study design of this study is rigorous and innovative.
The pain after abdominal surgery mainly comes from abdominal wall pain, which can last for 2–3 days [16]. Therefore, good abdominal wall analgesia is particularly important. According to studies, patients who utilized TAPB in conjunction with general analgesia could reduce opioid consumption by 36% [17]. TAPB combined with intravenous opioid analgesia was not inferior to thoracic epidural analgesia in the pain effects after abdominal surgery, but the incidence of postoperative nausea, vomiting, intestinal obstruction, and paresthesia was lower than that of thoracic epidural analgesia [18]. The duration of a single-injection TAPB generally does not exceed 12 h [19]. But the C-STAPB procedure used a long-term bilateral catheter and repeated intermittent injections of ropivacaine, which greatly prolonged the analgesic effect [20, 21]. In this study, there was no significant difference in the pain score at rest and movement, Moreover, the number of patients that require rescue analgesia between the C-STABP group and the conventional group indicates that there was no significant difference in the analgesic effect between the two groups. C-STAPB can achieve effective postoperative pain management with opioid-free.
Here, we demonstrate that C-STAPB is beneficial to the postoperative recovery of patients compared to the conventional group and reduces postoperative opioid-related adverse reactions. Our results showed that the C-STAPB group had earlier bowel recovered function in aspects of bowel movement, first defecation, and food intake compared to the conventional group. The fundamental reason for this result is that the patients in the C-STAPB group did not use opioids after surgery [4, 17]. In contrast to Stoner et al.’s findings, neither the incidence of dizziness nor the time off-bed differed significantly between the C-STAPB group and the conventional group [17]. Additionally, the C-STAPB group had a shorter length of stay in the hospital after surgery than the conventional group; the difference was not statistically significant. This occurrence may be caused by the fact that these indicators are strongly influenced by the patients’ and researchers’ own subjective personal variables.
The most common complications associated with TSAPB involve injection needles and regional anesthetics procedure. According to reports, situating the lower transverse abdominis plane block using body surface anatomical landmarks can accidentally penetrate the abdominal cavity and might injure the visceral organs [22]. With the development and application of ultrasound technology, the incidence of adverse events such as local hematoma and abdominal organ injury has been greatly reduced. Regional anesthetic-related complications of STAPB include seizures from local anesthetic systemic toxicity (LAST), ventricular arrhythmias, and transient nerve paralysis [23, 24]. When the block is required a high volume of local anesthetic drugs, a low concentration with an increased volume of drug should be considered for regional anesthesia. Therefore, ropivacaine, which has little effect on the central nervous system and cardiovascular and cerebrovascular system, is selected as a regional anesthetic, and it is less likely to cause adverse reactions such as hypertension, cardiac arrest, respiratory depression, and convulsions [25].
Since the analgesic effect of STAPB is based on the degree of spreading of the local anesthetic drug, the volume of the local anesthetic drug is the primary factor affecting the process of the block [25]. Studies have shown that there wasno advantage of increasing the local anesthetic drug greater than 20 ml for STAPB [25]. At present, there are two main methods of administration for C-STAPB: one is low-dose continuous infusion, and the other is repetitive intermittent administration [26, 27]. Compared with continuous infusion, an intermittent bolus of ropivacaine was found to not only lessen the overall local anesthetic requirement per day, but also has a wider sensory dermatome blockade and longer duration of analgesia.
The current research has several limitations. First of all, C-STAPB is troublesome to implement and requires good patient compliance. We will further simplify the procedure. Secondly, only patients undergoing laparoscopic radical gastrectomy for gastric cancer were included in this study. Whether these findings can be extended to other abdominal surgery remains to be evaluated. Thirdly, in the course of the experiment, three patients did withdraw from the experiment due to catheter displacement (6%), and the way of catheterization should be improved in further experiments. Fourthly, the study did not use patients control analgesia to measure the exact rescue analgesic requirement. The prn dose of rescue analgesic requirement by the nurses might be some barrier between patients and nurses or some factors that cause patients to not receive rescue analgesic drug. Finally, this is a single-center randomized controlled trial, and future studies with multiple centers and larger sample sizes are needed to verify the results of the study.
Conclusion
In conclusion, our results suggest that C-STAPB is not inferior to traditional opioid-based postoperative pain management for patients undergoing laparoscopic radical gastrectomy, suggesting that it is feasible to achieve complete opioid-free postoperative pain management. C-STAPB is a safe and feasible method with better sequelae, which accelerates the postoperative recovery of patients undergoing laparoscopic radical gastrectomy.
Availability of data and materials
Data are available with publication from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Mouton WG, Bessell JR, Otten KT, Maddern GJ. Pain after laparoscopy. Surg Endosc. 1999;13(5):445–8.
Simpson JC, Bao X, Agarwala A. Pain management in enhanced recovery after surgery (ERAS) protocols. Clin Colon Rectal Surg. 2019;32(2):121–8. https://doi.org/10.1055/s-0038-1676477.
Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402–12. https://doi.org/10.1007/s11999-015-4173-5.
Mittal T, Dey A, Siddhartha R, Nali A, Sharma B, Malik V. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postoperative analgesia in laparoscopic gastric sleeve resection: a randomized single blinded case control study. Surg Endosc. 2018;32(12):4985–9. https://doi.org/10.1007/s00464-018-6261-6.
Riemma G, Schiattarella A, Cianci S, La Verde M, Morlando M, Sisti G, et al. Transversus abdominis plane block versus wound infiltration for post-cesarean section analgesia: a systematic review and meta-analysis of randomized controlled trials. Int J Gynaecol Obstet. 2021;153(3):383–92. https://doi.org/10.1002/ijgo.13563.
El-Dawlatly AA, Turkistani A, Kettner SC, Machata AM, Delvi MB, Thallaj A, et al. Ultrasound-guided transversus abdominis plane block: description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102(6):763–7. https://doi.org/10.1093/bja/aep067.
Yoshida T, Furutani K, Watanabe Y, Ohashi N, Baba H. Analgesic efficacy of bilateral continuous transversus abdominis plane blocks using an oblique subcostal approach in patients undergoing laparotomy for gynaecological cancer: a prospective, randomized, triple-blind, placebo-controlled study. Br J Anaesth. 2016;117(6):812–20.
Ganapathy S, Sondekoppam RV, Terlecki M, Brookes J, Das Adhikary S, Subramanian L. Comparison of efficacy and safety of lateral-to-medial continuous transversus abdominis plane block with thoracic epidural analgesia in patients undergoing abdominal surgery: A randomised, open-label feasibility study. Eur J Anaesthesiol. 2015;32(11):797–804. https://doi.org/10.1097/EJA.0000000000000345.
Hahn S. Understanding noninferiority trials. Korean J Pediatr. 2012;55(11):403–7. https://doi.org/10.3345/kjp.2012.55.11.403.
Quartagno M, Carpenter JR, Walker AS, Clements M, Parmar MK. The DURATIONS randomised trial design: Estimation targets, analysis methods and operating characteristics. Clin Trials. 2020;17(6):644–53. https://doi.org/10.1177/1740774520944377.
Lee JS, Vu JV, Edelman AL, Gunaseelan V, Brummett CM, Englesbe MJ, et al. Health care spending and new persistent opioid use after surgery. Ann Surg. 2020;272(1). https://doi.org/10.1097/SLA.0000000000003399.
Ruiz-Tovar J, Muñoz JL, Gonzalez J, Zubiaga L, García A, Jimenez M, et al. Surg Endosc. 2017;31(1):231–6. https://doi.org/10.1007/s00464-016-4961-3.
Kikuchi S, Kuroda S, Nishizaki M, Matsusaki T, Mitsuhashi T, Kuwada K, et al. Randomized controlled trial of epidural versus patient-controlled intravenous analgesia for postoperative pain control after laparoscopic gastrectomy. Acta Med Okayama. 2018;72(1):95–8. https://doi.org/10.18926/AMO/55671.
Huang Z, Xie Y, You J, Xu L, Chen Y, Chen B, et al. Clinical significance of ropivacaine local infiltration for postoperative analgesia after total laparoscopic radical gastrectomy in elderly patients. Zhonghua Yi Xue Za Zhi. 2014;94(28):2188–92.
Cornish P, Deacon A. Rectus sheath catheters for continuous analgesia after upper abdominal surgery. ANZ J Surg. 2007;77(1–2):84.
Warren JA, Carbonell AM, Jones LK, McGuire A, Hand WR, Cancellaro VA, et al. Length of stay and opioid dose requirement with transversus abdominis plane block vs epidural analgesia for ventral hernia repair. J Am Coll Surg. 2019;228(4):680–6. https://doi.org/10.1016/j.jamcollsurg.2018.12.017.
Pirrera B, Alagna V, Lucchi A, Berti P, Gabbianelli C, Martorelli G, et al. Transversus abdominis plane (TAP) block versus thoracic epidural analgesia (TEA) in laparoscopic colon surgery in the ERAS program. Surg Endosc. 2018;32(1):376–82. https://doi.org/10.1007/s00464-017-5686-7.
Abdallah FW, Laffey JG, Halpern SH, Brull R. Duration of analgesic effectiveness after the posterior and lateral transversus abdominis plane block techniques for transverse lower abdominal incisions: a meta-analysis. Br J Anaesth. 2013;111(5):721–35. https://doi.org/10.1093/bja/aet214.
Sondekoppam RV, Brookes J, Morris L, Johnson M, Ganapathy S. Injectate spread following ultrasound-guided lateral to medial approach for dual transversus abdominis plane blocks. Acta Anaesthesiol Scand. 2015;59(3):369–76. https://doi.org/10.1111/aas.12459.
Petersen PL, Hilsted KL, Dahl JB, Mathiesen O. Bilateral transversus abdominis plane (TAP) block with 24 hours ropivacaine infusion via TAP catheters: a randomized trial in healthy volunteers. BMC Anesthesiol. 2013;13(1):30. https://doi.org/10.1186/1471-2253-13-30.
O'Donnell BD, Mannion S. A case of liver trauma with a blunt regional anesthesia needle while performing transversus abdominis plane block. Reg Anesth Pain Med. 2009;34(1). https://doi.org/10.1097/AAP.0b013e3181934050.
Scherrer V, Compere V, Loisel C, Dureuil B. Cardiac arrest from local anesthetic toxicity after a field block and transversus abdominis plane block: a consequence of miscommunication between the anesthesiologist and surgeon. A A Case Rep. 2013;1(5):75–6. https://doi.org/10.1097/ACC.0b013e3182973a3f.
Naidu RK, Richebe P. Probable local anesthetic systemic toxicity in a postpartum patient with acute fatty liver of pregnancy after a transversus abdominis plane block. A A Case Rep. 2013;1(5):72–4. https://doi.org/10.1097/ACC.0b013e3182973a2f.
Bao X, Huang J, Feng H, Qian Y, Wang Y, Zhang Q, et al. Effect of local anesthetic volume (20 mL vs 30 mL ropivacaine) on electromyography of the diaphragm and pulmonary function after ultrasound-guided supraclavicular brachial plexus block: a randomized controlled trial. Reg Anesth Pain Med. 2019;44(1):69–75. https://doi.org/10.1136/rapm-2018-000014.
Rao Kadam V, Van Wijk RM, Moran JL, Ganesh S, Kumar A, Sethi R, et al. Continuous transversus abdominis plane block vs intermittent bolus for analgesia after abdominal surgery: a randomized trial. J Pain Res. 2017;10:1705–12. https://doi.org/10.2147/JPR.S132891.
Khatibi B, Said ET, Sztain JF, Monahan AM, Gabriel RA, Furnish TJ, et al. Anesth Analg. 2017;124(4):1298–303. https://doi.org/10.1213/ANE.0000000000001939.
Acknowledgements
We thank all the patients who participated in this study.
Funding
The foundation of the 900th Hospital of Joint Logistic Support Force, PLA (2021MS13).
Author information
Authors and Affiliations
Contributions
ZML and ZBC designed the study; ZML performed the statistical analyses and generated figures and tables; YYL wrote the manuscript; all authors have agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This trial was approved by the institutional review board of the 900th Hospital of Joint Logistic Support Force, PLA (2014046). All participants provided written consent to participate.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Z., Chen, Z. & Li, Y. Analgesic efficacy of an opioid-free postoperative pain management strategy versus a conventional opioid-based strategy following laparoscopic radical gastrectomy: an open-label, randomized, controlled, non-inferiority trial. World J Surg Onc 22, 54 (2024). https://doi.org/10.1186/s12957-023-03298-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03298-x