Abstract
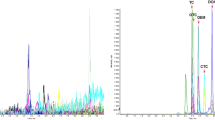
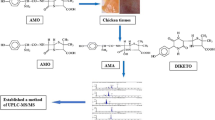
Florfenicol is a chloramphenicol antibiotic that plays an essential role on bacteriostasis. Long-term use of florfenicol in livestock and poultry can cause immunetoxicity and reproductive toxicity and the consumption of animal-derived products with excessive residues of florfenicol will pose a certain threat to human health. To study residue depletion of oral florfenicol in chickens, 48 healthy 30 -day -old AA broilers received continuous administration of 150 mg/kg/d florfenicol for five days. Muscle, liver, kidney and sebum were collected at 0.16, 1, 3, 5, 7 and 9 days after discontinuation of the drug and detected by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Most previous studies have shown that acid hydrolysis is necessary for tissue sample extraction. However, the steps of acid hydrolysis are tedious and time-consuming. In this experiment, we tried to improve the solvent extraction method to simplify the pretreatment process and the effect of acid hydrolysis was further analyzed and compared. Using the WT1.4 software the withdrawal time in muscle, liver, kidney and sebum obtained by F-QuEChERS-AOAC 3202 method was 7.82, 0.06, 4.38, 13.71 days respectively while acid hydrolysis method were 8.89, 5.84, 5.39 and 9.65 days, respectively. To reduce the residues of florfenicol and ensure the safety of chicken products, it is recommended that chickens taking oral florfenicol should be subjected to a 14 -days withdrawal time.




Similar content being viewed by others
References
Myung-Jin Choia, Eun-Mi Leea, Seung-Jin Lee, Md. Ahsanur Reza, Man-Hee Rhee and Seung-Chun Park (2011). Pak Vet J. doi: https://doi.org/10.1080/00480169.2011.610285
Yu. Ying Wang, Z.L. An, Yu. Zhou, D. Zhang, Food Addit Contama (2020). https://doi.org/10.1080/19440049.2019.1591641
K.N. Bretzlaff, C.A. Neff-Davis, R.S. Ott, G.D. Koritz, L.E. Davis, J Vet Pharmacol Ther (2010). https://doi.org/10.1111/j.1365-2885.1987.tb00534.x
A. Sieroslawska, M. Studnicka, A. Bownik, A. Rymuszka, J. Slonka, A.K. Siwicki, Acta Vet Brno (1998). https://doi.org/10.2754/avb199867040329
G.X. Yang, G.D. Deng, M. Zou, Z.L. Zeng, Z.L. Chen, Acute and Subchronic Toxicity of Florfenicol on Rats. Chinese Journal of Veterinayr Science 03, 275–278 (2000)
R.H. Abubakar, E. Madoroba, O. Adenubi, D. Morar-Leather, F.O. Fasina, J Vet Med Anim Health (2017). https://doi.org/10.5897/JVMAH2017.0594
L. Sun, L. Zhang, Q. Xu, X. Wang, S. Wang, Research development of toxicity and residue detection methods of florfenicol. Chin J Vet Drug 43(6), 49–52 (2009)
H.M. Jang, Y.B. Kim, S. Choi, Y. Lee, S.G. Shin, T. Unno et al., Environ Pollut (2016). https://doi.org/10.1016/j.envpol.2017.10.006
Z. Ping, P. Linyao, X.U. Changwen, L.I. Yunxia, Z. Anyun, Study on Florfenicol Resistance and fexA Genetic Environment in Enterococcus from Swine. China Animal Husbandry 45(6), 1700–1707 (2018)
F.D. Wu, Florfenicol Resistance and Sequence Analysis of floR Gene in Different Bacteria. Chin. Agric. Sci. Bull. 35(2), 122–126 (2018)
S. Schwarz, C. Werckenthin, C. Kehrenberg, Antimicrob Agents Ch (2000). https://doi.org/10.1128/AAC.44.9.2530-2533.2000
W. Tao, M.H. Lee, J. Wu, N.H. Kim, J.C. Kim, E. Chung et al., Appl Environ Microbiol (2012). https://doi.org/10.1128/AEM.01154-12
C. Kehrenberg, S. Schwarz, Antimicrob Agents Ch (2004). https://doi.org/10.1128/AAC.48.2.615-618.2004
L. Hebing, W. Yang, W. Congming, S. Stefan, S. Zhangqi, J. Byeonghwa, J Antimicrob Chemoth (2012). https://doi.org/10.1093/jac/dkr481
E.H. Kim, T. Aoki, Microbiol Immunol (1996). https://doi.org/10.1111/j.1348-0421.1996.tb01125.x
Y.H. Wang, G.Q. Wang, Microb Pathogesis (2018). https://doi.org/10.1016/j.micpath.2018.02.042
K.S. Lang, J.M. Anderson, S. Schwarz, L. Williamson, J. Handelsman, R.S. Singer, Appl Environ Microb (2010). https://doi.org/10.1128/AEM.00323-10
H. Tao, S. Jianzhong, S. Stefan, W. Congming, W. Yang, J Antimicrob Chemoth (2015). https://doi.org/10.1093/jac/dku491
Da Costa, P. , Loureiro, Luís, & Matos, A. . (2013). Int. J. Environ. Res. Public Health. Doi: https://doi.org/10.3390/ijerph10010278
M.G. Brenner, K. Kristina, M.T. Sweeney, B. Elzbieta, L. Heiko, D. Rolf, J Antimicrob Chemoth (2012). https://doi.org/10.1093/jac/dkr406
S. Jianzhong, W. Yang, S. Stefan, J Antimicrob Chemoth (2013). https://doi.org/10.1093/jac/dkt092
A. Nasim, B. Aslam, I. Javed, A. Ali, F. Muhammad, A. Raza, J Sci Food Agr. (2015). https://doi.org/10.1002/jsfa.7220
State Administration for Market Regulation. Notification of 24 batches of disqualified food [EB/OL]. [2018–10–23]. http://samr.saic.gov.cn/tg/201810/t20181023_276376.html.
Shanoy, C., Anderson, Seenivasan, Subbiah, & Angella, et al. (2016). J Chromatogr B. doi: https://doi.org/10.1016/j.jchromb.2016.08.014
L.J.M. Jansen, R.J. Berentsen, M. Arends, B.J.A. Berendsen, Food Addit Contama A. (2020). https://doi.org/10.1080/19440049.2020.1725147
P.P.J. Mulder, S.L.D. Witte, G.M. Stoopen, J.V.D. Meulen, P.G.V. Wikselaar, E. Gruys, Food Addit Contama A (2016). https://doi.org/10.1080/19440049.2016.1241430
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin, Off. J. of Eur. Union. L 15 (2010) 1-72.
S. Al-Shahrani, V. Naidoo, BMC Vet Res. (2015). https://doi.org/10.1186/s12917-015-0536-0
Afifi, N. A. , & El‐Sooud, K. Abo. (1997). Br Poult. Doi: https://doi.org/10.1080/00071669708418013
Committee for Veterinary Medicinal Products: Florfenicol, Summary Report(1). http://www.eudra. Org/ emea. Html. 2002, (1)
Notice No. 235 of the Ministry of Agriculture of the people's Republic of China《National food safety standard-Maximum residue limits for veterinary drugs in foods》.2019.
D. Faulkner, M. Cantley, M. Walker, S. Crooks, D. Kennedy, C. Elliott, Food Addit Contam A. (2016). https://doi.org/10.1080/19440049.2016.1175187
Muhammad Imran, Fazal-e-Habib, Abdul Tawab, Waqar Rauf, Moazur Rahman. (2017). J Chromatogra B. doi: https://doi.org/10.1016/j.jchromb.2017.08.029.
A. Kong, A. Deng, X. Wu, J. Qiao, J Liq Chromatogr R T. (2014). https://doi.org/10.1080/10826076.2013.864976
Animal management regulations (2016). Electronic Journal of Practical Organ Transplantation.; 4(2),66–67.
M.P.G. Nahler, Committee for Veterinary Medicinal Products (CVMP) (2009). https://doi.org/10.1007/978-3-211-89836-9_245
Commission E (2002) Commission Decision (EC) no 675/2002 of 12th August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Union. L221/8–36.
F.S. Aliabadi, P. Lees, Int J Antimicrob Ag (2000). https://doi.org/10.1016/S0924-8579(00)00142-4
Zhang Xu (2005). “Studies on the Post-antibiotic Effects and Pharmacokinrtics of Florfenicol in Chicken.” Huazhong Agricultural University.
M. Ismail, Y.A. El-Kattan, Brit Poultry Sci. (2009). https://doi.org/10.1080/00071660802613286
J. Cornejo, E. Pokrant, R. Riquelme, C. Briceno, A. Maddaleno, C. Araya-Jordan, Food Addit Contam. (2017). https://doi.org/10.1080/19440049.2016.1267876
N.B. Po, O.P. Paw, A.U. Pas, T. Grabowski, A. Suszko, M. Lis, Brit Poultry Sci. (2017). https://doi.org/10.1080/00071668.2016.1261994
F.A. Mohammed, Comparison of three nonlinear functions for describing chicken growth curves. Scientia Agriculturae 9(3), 120–123 (2015)
J.B. Feng, D.R. Huang, M. Zhong, P. Liu, J.D. Dong, J Fish Dis. (2016). https://doi.org/10.1111/jfd.12416
H. Ekici, E. Yarsan, J Anim Vet Adv. (2014). https://doi.org/10.5455/javar.2014.a28
H.A. El-Banna, Brit Poultry Sci. (1998). https://doi.org/10.1080/00071669888656
Lu Y. Z. (2007) “The Determination Method of ThiamPhenicol and F1orfenieol Residues and their Elimination in Chicken Tissues.” Anhui Agricultural University.
B.J.A. Berendsen, G. Bor, H.W. Gerritsen, L.J.M. Jansen, T. Zuidema, Food Addit Contam A. (2013). https://doi.org/10.1080/19440049.2013.843026
B. San Martín, J. Cornejo, D. Iraguen, H. Hidalgo, A. Anadón, J Food Protect. (2007). https://doi.org/10.1111/j.1745-4549.2007.00132.x
L.J.M. Jansen, Y.J.C. Bolck, J. Rademaker, T. Zuidema, B.J.A. Berendsen, Anal Bioanal Chem. (2017). https://doi.org/10.1007/s00216-017-0445-0
Acknowledgment
This work was supported by the Shandong Key R & D Plan (Major Scientific and Technological Innovation Project)-(2019JZZY020903) and Shandong Academy of agricultural sciences agricultural science and technology innovation engineering industry-major technological innovation (CXGC2016B17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, Y., Fan, L., zhao, L. et al. Development and validation of a method for quantification of residual florfenicol in various tissues of broiler chicken by UPLC-MS/MS. Food Measure 15, 3143–3152 (2021). https://doi.org/10.1007/s11694-021-00874-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00874-1