Abstract
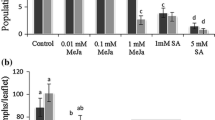
To study the effect of jasmonates (JAs) on the resistance of economic forest plants to insects, Rosa rugosa ‘Plena’ leaves were treated with 1 mmol/L jasmonic acid (JA), methyl jasmonate (MeJA) and Z-jasmone, then the content of tannin and total phenol in leaves and the feeding area of Monolepta hieroglyphica adults on leaves were measured. Changes in the activities of detoxification enzymes in adult M. hieroglyphica that had fed on leaves treated with JAs were also studied. Tannin and total phenol levels in leaves increased significantly after treatment with JAs, and tannin level was 1.36–1.55-fold higher than in the control after treatment with 1 mmol/L MeJA. The total content of phenol in leaves treated with 1.0 mmol/L Z-jasmone increased by 1.33–2.20-fold compared with those of the control. The activities of detoxification enzymes in adults were inhibited to differing degrees: activity of alkaline phosphatase (AKP) first increased, then decreased; the activities of acid phosphatase (ACP), glutathione S-transferases (GSTs) and carboxylesterase (CarE) following treatment with 1 mmol/L MeJA were significantly reduced and were 22%–31%, 11%–26%, and 11%–31% lower than those of the control, respectively. Moreover, the feeding area of adult M. hieroglyphica on the leaves treated with JAs was significantly reduced (P < 0.05). The feeding area of economic forest R. rugosa ‘Plena’ leaves treated with 1 mmol/L MeJA decreased by 17%–43% compared with that of the control. Moreover, the decrease in the adult M. hieroglyphica feeding area was highly positively correlated with the content of tannin and positively correlated with the contents of total phenol of economic forest R. rugosa ‘Plena’ leaves. The reduced feeding area of adult M. hieroglyphica was highly negatively correlated with the activities of AKP and ACP and negatively correlated with those of the GSTs. In conclusion, the use of 1 mmol/L MeJA can noticeably decrease the deleterious effects of adult M. hieroglyphica.



Similar content being viewed by others
References
Barbehenn RV, Constabel CP (2011) Tannins in plant-herbivore interactions. Phytochemistry 72:1551–1565
Bass C, Jones CM (2018) Editorial overview: Pests and resistance: Resistance to pesticides in arthropod crop pests and disease vectors: mechanisms, models and tools. Curr Opin Insect Sci. https://doi.org/10.1016/j.cois.2018.04.009
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem 164:321–329
Bilal M, Freed S, Ashraf MZ, Zaka SM, Khan MB (2018) Activity of acetylcholinesterase and acid and alkaline phosphatases in different insecticide-treated Helicoverpa armigera (Hübner). Environ Sci Polut Res 25:22903–22910
Bodnaryk RP, Rymerson RT (1994) Effect of wounding and jasmonates on the physico-chemical properties and flea beetle defence responses of canola seedlings, Brassica napus L. Can J Plant Sci 74:899–907
Boulogne I, Petit P, Ozier-Lafontaine H, Destontainers L, Loranger-Merciris G (2012) Insecticidal and antifungal chemicals produced by plants: a review. Environ Chem Lett 10:325–347
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(12):248–254
Cai QN, Han Y, Cao YZ, Hu Y, Zhao X, Bi JL (2009) Detoxification of gramine by the cereal aphid Sitobion avenae. J Chem Ecol 35:320–325
Chang K, Shi YX, Chen JQ, He ZH, Xu Z, Zhao ZJ, Zhu WP, Li HL, Xu YF, Li BJ, Qian XH (2016) The discovery of new plant activators and scaffolds with potential induced systemic resistance: from jasmonic acid to pyrrolidone. Med Chem Commun. https://doi.org/10.1039/C6MD00261G
Chang SW, Du YE, Qi YT, Lee JS, Goo N, Koo BK, Bae HJ, Ryu JH, Jang DS (2019) New depsides and neuroactive phenolic glucosides from the flower buds of Rugosa Rose (Rosa rugosa). J Agric Food Chem 67(26):7289–7296
Chen FJ, Gao XW, Lei MQ, Zheng BZ (2003) Effect of tannic acid on glutathione S transferases in Helicoverpa armigera (Hübner). Acta Entomol Sin 46(6):684–690 (in Chinese)
Chen J, Zhang JP, Zhang JH, Yu HF, Li GW (2007) Food preference of Monolepta hieroglyphica. Chin Bull Entomol 44(3):357–360 (in Chinese)
Cooper WR, Rieske LK (2008) Differential responses in American (Castanea dentata Marshall) and Chinese (C. mollissima Blume) chestnut (Falales: Fagaceac) to foliar application of jasmonic acid. Chemoecology 18:121–127
Cui F, Lin Z, Wang HS, Liu SL, Chang HJ, Reeck G, Qiao CL, Raymood M, Kang L (2011) Two single mutations commonly cause qualitative change of nonspecific carboxylesterases in insects. Insect Biochem Mol Biol 41(1):1–8
Dam NMV, Hadwich K, Baldwin IT (2000) Induced responses in Nicotiana attenuata affect behavior and growth of the specialist herbivore Manduca sexta. Oecologia 122:371–379
Delaney KJ, Wawrzyniak M, Lemanczyk G, Wrzesinska D, Piesik D (2013) Synthetic cis-jasmone exposure induces wheat and barley volatiles that repel the pest cereal leaf beetle. Oulema melanopus L J Chem Ecol 39(5):620–629
Despres L, David JP, Gallet C (2007) The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol Evol 22:298–307
Dixit G, Praveen A, Tripathi T, Yadav VK, Verma PC (2017) Herbivore-responsive cotton phenolics and their impact on insect performance and biochemistry. J Asia-Pac Entomol 20(2):341–351
Falk KL, Kistner J, Bodenhausen N, Schramm K, Paetz C, Vassao DG, Reichelt M, Knorre DV, Bergelson J, Erb M, Gershenzon J, Meldau S (2014) The role of glucosinolates and the jasmonic acid pathway in resistance of Arabidopsis thaliana against molluscan herbivores. Mol Ecol 23:1188–1203
Feng QL, Davey KG, Pang ASD, Ladd TR, Retnakaran A (2001) Developmental expression and stress induction of glutathione S-transferases in the spruce budworm, Choristoneura fiumiferana. J Insect Physiol 47:1–10
Firake DM, Thubru DP, Behere GT (2017) Eco-toxicological risk and impact of pesticides on important parasitoids of cabbage butterflies in cruciferous ecosystem. Chemosphere 168:372–383
Florian S, Andreas S, Annick S (2005) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23:179–199
Franciosa H, Berge JB (1995) Glutathione-S-transferases in housefly (Musca domestica): location of GST-1 and GSH-2 families. Insect Biochem Mol Biol 25:311–317
Francis F, Vanhaelen N, Haubruge E (2005) Glutathione S-transferases in the adaptation to plant secondary metabolites in the Myzus persicae aphid. Arch Insect Biochem Physiol 58(3):166–174
Gracen JVE, Guthrie WD (1986) Host plant resistance for insect control in some important crop plants. Crit Rev Plant Sci 4(3):277–291
Gui LY, Chen ZM, Liu SS (2005) Effects of exogenous MJA treatment of tea plants on the growth of geometrid larvae. Sci Agric Sin 58:203–307
Guo DD, Zhang ZH, Chen J, Wang SS (2018) Electrophysiological and behavioral responses of Monolepta hieroglyphica (Motschulsky) to 7 cotton and com volatiles. J App Entomol 55(1):79–86 (in Chinese)
Hu ZJ, Shao SJ, Zheng CF, Sun ZH, Shi JY, Yu JQ, Qi ZY, Shi K (2018) Induction of systemic resistance in tomato against Botrytis cinerea by N-decanoyl-homoserine lactone via jasmonic acid signaling. Planta 247:1217–1227
Ibrahim S, Mir GM, Rouf A, War AR, Hussaina B (2018) Herbivore and phytohormone induced defensive response in kale against cabbage butterfly, Pieris brassicae Linn. J Asia-Pac Entomol 21:367–373
Jafari M, Minaei S, Safaie N (2017) Detection of pre-symptomatic rose powdery-mildew and gray-mold diseases based on thermal vision. Infrared Phys Technol 85:170–183
Jiang D, Meng ZJ, Yan SC (2017) Effects of partially spraying Larix olgensis seedlings with exogenous methyl jasmonate on the defensive enzyme activities of Dendrolimus superans larvae. J Beijing For Univ 39(2):58–63 (in Chinese)
Kaur H, Salh PK, Singh B (2017) Role of defense enzymes and phenolics in resistance of wheat crop (Triticum aestivum L.) towards aphid complex. J Plant Interact 12:304–311
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Sci 291:2141–2144
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Ketabchi S, Majzoob SH, Charegani HA (2014) Effect of salicylic acid and methyl jasmonate on phenylalanine ammonia-lyase activity and total phenol in wheat infected by Pratylenchus thornei. Arch Phytopathol Plant Prot 48(1):10–17
Koo AJK, Howe GA (2009) The wound hormone jasmonate. Phytochemistry 70(13–14):1571–1580
Ku CC, Chiang FM, Hsin CY, Yao YE, Sun CN (1994) Glutathione transferases isozymes involved in insecticide resistance of diamondback moth larvae. Pestic Biochem Physiol 50:191–197
Li ZJ, Zhao MY, Jin JF, Zhao LY, Xu ZD (2018) Anthocyanins and their biosynthetic genes in three novel-colored Rosa rugosa cultivars and their parents. Plant Physiol Biochem 129:421–428
Macel M, Visschers IGS, Peters JL, Kappers IF, de Vos RCH, van Dam NM (2019) Metabolomics of thrips resistance in pepper (Capsicum spp.) reveals monomer and dimer acyclic diterpene glycosides as potential chemical defenses. J Chem Ecol 45:490–501
Maciag A, Kalemba D (2015) Composition of rugosa rose (Rosa Rugosa, Thunb.) hydrolate according to the time of ditllation. Phytochem Lett 11:373–377
Matsuki S, Sano Y, Koike T (2004) Chemical and physical defence in early and late leaves in three heterophyllous birch species native to northern Japan. Ann Bot 93(2):141–147
Pauwels L, Inzé D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 14(2):87–91
Pickett JA, Birkett MA, Bruce TJA, Chamberlain K, Gordon-Weeks R, Matthes MC, Napier JA, Smart LE, Woodcock CM (2007) Developments in aspects of ecological phytochemistry: the role of cis-jasmone in inducible defence systems in plants. Phytochemistry 68(22–24):2937–2945
Prapanthadara L, Promter N, Kottathep S, Somboon P, Ketterman AJ (2000) Isoenzymes of glutathione S-transferase from the mosquito Anopheles dirus species B: the purification, partial characterization and interaction with various insecticides. Insect Biochem Mol Biol 30:39–403
Rani UP, Jyothsna Y (2010) Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiol Plant 32:695–701
Robert EB (1971) Method for estimation of tannin in grain sorghum. Agron J 63:511–512
Rusanov K, Kovacheva N, Rusanova M, Linde M, Debener T, Atanassov T (2019) Genetic control of flower petal number in Rosa x Damascena Mill. f. trigintipetala. Biotechnol Biotechnol Equip 33(1):597–604
Senthil-Nathan S (2019) Effect of methyl jasmonate (MeJA)-induced defenses in rice against the rice leaffolder Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae). Pest Manag Sci 75(2):460–465
Senthil-Nathan S, Kalaivani K, Murugan K, Chung PG (2005) The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guenée) the rice leaffolder. Pestic Biochem Physiol 81(2):113–122
Senthil-Nathan S, Kalaivani K, Sehoon K, Murugan K (2006) The toxicity and behavioural effects of neem limonoids on Cnaphalocrocis medinalis (Guenée), the rice leaffolder. Chemosphere 62(8):1381–1387
Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD (2001) Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci 98(8):4788–4793
Sharma HC, Sujana G, Rao DM (2009) Morphological and chemical components of resistance to pod borer Helicoverpa armigera in wild relatives of pigeonpea. Arthropod-Plant Interact 3:151–161
Sheng LX, Zeng YQ, Wei TT, Zhu M, Fang XM, Yuan XY, Luo YJ, Feng LG (2018) Cloning and functional verification of genes related to 2-phenylethanol biosynthesis in Rosa rugosa. Genes (Basel) 9(12):576
Sripontan Y, Hwang SY (2016) Jasmonate-induced defense in tomato and cabbage deterred Spodoptera litura (Noctuidae) growth. J Asia-Pac Entomol 19(4):1125–1129
Tang JX, Yang DH, Wu JQ, Chen SY, Wang L (2020) Silencing JA hydroxylases in Nicotiana attenuata enhances jasmonic acid-isoleucine-mediated defenses against Spodoptera litura. Plant Divers 42(2):111–119
Tebayashi S, Horibata Y, Mikagi E, Kashiwagi T, Mekuria DB, Dekebo A, Ishihara A, Kim CS (2007) Induction of resistance against the leafiminer, Liriomyza trifolli, by jasmonic acid in sweet pepper. Biosci Biotechnol Biochem 71(6):1521–1526
Thaler JS, Fidantsef AL, Duffey SS, Bostock RM (1999) Trade-offs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. J Chem Ecol 25:1597–1609
Tian J, Cui J, Wu L, Xu W, Chen BC, Shi SS (2014) Screening of pesticides for controlling Monolepta hieroglyphica (Motschulsky). Agrochem 53(10):767–770 (in Chinese)
Van Asperen K (1962) A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol 8:401–416
Vishwanathan K, Zienkiewicz K, Liu Y, Janz D, Feussner I, Polle A, Haney CH (2020) Ectomycorrhizal fungi induce systemic resistance against insects on a non-mycorrhizal plant in a CERKl-dependent manner. New Phytol. https://doi.org/10.1111/nph.16715
Wang JB (2009) Monogalactosyldiacylglycerol deficiency affects jasmonic acid biosynthesis and defense responses to insect herbivores in Nicotiana tobacum. Plant Sci 176(2):279–285
War AR, Paularj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7(10):1306–1320
War AR, Paulraj MG, War MY, Ignacimuthu S (2011) Jasmonic acid-mediated induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J Plant Growth Regul 30:512–523
Wasternack C, Stmad M (2015) Jasmonate signaling in plant stress responses and development: active and inactive compounds. New Biotechnol 33:604–613
Yan JX, Deng YN, Yu J, Zhang YQ, Chi DF (2018) A study on JA- and BTH-induced resistance of Rosa rugosa “Plena” to powdery mildew (Sphaerotheca pannosa). J For Res 29:823–831
Yan JX, Xu LX, Yu J, Zhang YQ, Chi DF (2017) Effects of MeJA on the insect-resistant physiological indexes of Rosa rugosa “Plena” and the feeding of Monolepta hieroglyphica. J Northeast For Univ 45(1):77–81 (in Chinese)
Yang S, Wu H, Xie J, Rantala MJ (2013) Depressed performance and detoxification enzyme activities of Helicoverpa armigera fed with conventional cotton foliage subjected to methyl jasmonate exposure. Entomol Exp Appl 147(2):186–195
Yang XM, Margolies DC, Zhu KY, Buschman LL (2001) Host plant-induced changes in detoxification enzymes and susceptibility to pesticides in the two spotted spider mite (Acar: Tetranychidac). J Econ Entomol 94(2):381–387
Zas R, Bjorklund N, Nordlander G, Cendan C, Hellqvist C, Sampedro L (2014) Exploiting jasmonate-induced responses for field protection of conifer seedlings against a major forest pest. Hylobius abietis For Ecol Manag 313(1):212–223
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was supported financially by the National Natural Science Foundation of China (No. 31800546), the National Key R & D Program of China (No. 2018YFC1200400), and the Fundamental Research Funds for the Central Universities (No. 2572016CA11).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Rights and permissions
About this article
Cite this article
Yan, J., Tan, Y., Lv, Y. et al. Effect of jasmonate treatments on leaves of Rosa rugosa ‘Plena’ and detoxification enzymes and feeding of adult Monolepta hieroglyphica. J. For. Res. 32, 1253–1261 (2021). https://doi.org/10.1007/s11676-020-01278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01278-5