Abstract
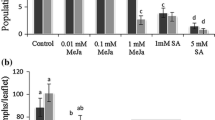
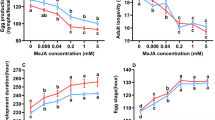
Methyl jasmonate (MeJA) is a plant chemical elicitor that has been used to artificially induce chemical defense responses and trigger induced resistance against a broad range of arthropod herbivores. This study assessed the effects of exogenous MeJA on the growth performance, chemical detoxification, and antioxidant enzyme activities of Clostera anachoreta. After feeding C. anachoreta with 10−5 mol/L MeJA solution-treated Populus × euramericana ‘Nanlin895’ leaves, we measured the larval and pupal development time, pupal weight, eclosion rate, fecundity, and nutritional physiology of the adults. We also measured superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) activities, which are reactive oxygen species (ROS) scavengers, and glutathione S-transferase (GST) and carboxylesterase (CarE) activities, which are probably involved in the metabolism of induced plant allelochemicals. Methyl jasmonate (MeJA) treatment reduced larval performance in terms of prolonged developmental time of larvae and pupae and decreased growth rates, but had little effect on larval nutrition physiology. The activities of the SOD and POD antioxidant enzymes increased, but CAT activity declined at 36 and 48 h after C. anachoreta had fed on MeJA-treated leaves. The GST and CarE detoxification enzymes both were induced after the larvae had fed on MeJA-treated leaves. These results suggest that exogenous application of MeJA elicited induced resistance in Populus × euramericana ‘Nanlin895’ against C. anachoreta.

Similar content being viewed by others
References
Agrawal AA (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80(5):1713–1723
Ahmad S (1992) Biochemical defence of pro-oxidant plant allelochemicals by herbivorous insects. Biochem Ecol Syst 20:269–296
Ahmad S, Pardini RS (1990) Antioxidant defense of the cabbage looper, Trichoplusia ni: enzymatic responses to the superoxide-generating flavonoid, quercetin, and photodynamic furanocoumarin, xanthotoxin. Photochem Photobiol 15:305–311
Ahn JE, Salzman RA, Braunagel SC et al (2004) Functional roles of specific bruchid protease isoforms in adaptation to a soybean protease inhibitor. Insect Mol Biol 13:649–657
Asperen VK (1962) A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol 8(4):414–416
Ballaré CL (2011) Jasmonate-induced defenses: a tale of intelligence, collaborators and rascals. Trends Plant Sci 16(5):249–257
Barbehenn RV (2002) Gut-based antioxidant enzymes in a polyphagous and a graminivorous grasshopper. J Chem Ecol 28(7):1329–1347
Bass C, Field LM (2011) Gene amplification and insecticide resistance. Pest Manag Sci 67(8):886–890
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Carvalhais LC, Dennis PG, Badri DV et al (2013) Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS ONE 8(2):e56457
Champigny MJ, Cameron RK (2009) Action at a distance: long-distance signals in induced resistance. Adv Bot Res 51:123–171
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge
Degenhardt J, Gershenzon J, Baldwin IT, Kessler A (2003) Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol 14(2):169–176
Délano-Frier JP, Martínez-Gallardo NA, Martínez-de LVO et al (2004) The effect of exogenous jasmonic acid on induced resistance and productivity in amaranth (Amaranthus hypochondriacus) is influenced by environmental conditions. J Chem Ecol 30(5):1001–1034
Farmer EE, Ryan CA (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci 87(19):7713–7716
Fedderwitz F, Nordlander G, Ninkovic V et al (2016) Effects of jasmonate-induced resistance in conifer plants on the feeding behaviour of a bark-chewing insect, Hylobius abietis. J Pest Sci 89(1):97–105
Felton GW, Duffey SS (1991) Protective action of midgut catalase in lepidopteran larvae against oxidative plant defenses. J Chem Ecol 17(9):1715–1732
Felton GW, Summers CB (1995) Antioxidant systems in insects. Arch Insect Biochem Physiol 29:187–197
Feng YJ, Wang JW, Luo S, Fan H, Jin Q (2012) Costs of jasmonic acid induced defense in above ground and below ground parts of corn (Zea mays L.). J Chem Ecol 38(8):984–991
Graves AD, Holsten EH, Ascerno ME et al (2008) Protection of spruce from colonization by the bark beetle, Ips perturbatus, in Alaska. Forest Ecol Manag 256(11):1825–1839
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
Hemming JDC, Lindroth RL (2000) Effects of phenolic glycosides and protein on gypsy moth (Lepidoptera: Lymantriidae) and forest tent Caterpillar (Lepidoptera: Lasiocampidae) performance and detoxication activities. Environmental Entomology 29(6):1108–1115
Hu Z, Zhang W, Shen Y et al (2009) Activities of lipoxygenase and phenylalanine ammonia lyase in poplar leaves induced by insect herbivory and volatiles. J For Res 20(4):372–376
Kao CH, Hung CF, Sun CN (1989) Parathion and methyl parathion resistance in diamondback moth (Lepidoptera: Plutellidae) larvae. J Econ Entomol 82(5):1299–1304
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291(5511):2141–2144
Krishnan N, Kodrik D (2006) Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protect gut tissues during oxidative stress? J Insect Physiol 52:11–20
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52:231–253
Liang ZP, Zhang XX, Song AD et al (2006) Biology of Clostera anachoreta and its control methods. Chin Bull Entomol 43:147–152
Lindroth RL, Bloomer MS (1991) Biochemical ecology of the forest tent caterpillar: responses to dietary protein and phenolic glycosides. Oecologia 86(3):408–413
Liu Q, Zhou Y, Chen J et al (2015) Defensive responses of Populus deltoides 895 seedlings against exogenous methyl jasmonate. Pak J Bot 47(1):177–188
Lundborg L, Fedderwitz F, Björklund N et al (2016) Induced defenses change the chemical composition of pine seedlings and influence meal properties of the pine weevil Hylobius abietis. Phytochemistry 130:99–105
Lv M, Sun HH, Wang LH et al (2012) Effects of secondary metabolites on activities of glutathione S-transferases, carboxylesterase in aphid. Chin Agric Sci Bull 28(3):253–256
Martin JA, Solla A, Garcia-Vallejo MC et al (2012) Chemical changes in Ulmus minor xylem tissue after salicylic acid or carvacrol treatments are associated with enhanced resistance to Ophiostoma novo-ulmi. Phytochemistry 83:104–109
Mitrovic SM, Pflugmacher S, James KJ et al (2004) Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat Toxicol 68(2):185–192
Omer AD, Thaler JS, Granett J et al (2000) Jasmonic acid induced resistance in grapevines to a root and leaf feeder. J Econ Entomol 93(3):840–845
Poelman EH, Broekgaarden C, Van Loon JJA et al (2008) Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol Ecol 17(14):3352–3365
Reinbothe S, Reinbothe C, Lehmann J et al (1994) JIP60, a methyl jasmonate-induced ribosome-inactivating protein involved in plant stress reactions. Proc Natl Acad Sci 91(15):7012–7016
Rigsby CM, Showalter DN, Herms DA et al (2015) Physiological responses of emerald ash borer larvae to feeding on different ash species reveal putative resistance mechanisms and insect counter-adaptations. J Insect Physiol 78:47–54
Rodriguez-Saona C, Chalmers JA, Raj S et al (2005) Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia 143(4):566–577
Rohwer CL, Erwin JE (2008) Horticultural applications of jasmonates. J Hortic Sci Biotechnol 83(3):283–304
Schaller A, Stintzi A (2008) Jasmonate biosynthesis and signaling for induced plant defense against herbivory. Induced plant resistance to herbivory. Springer, Netherlands, pp 349–366
Scott IM, Thaler JS, Scott JG (2010) Response of a generalist herbivore Trichoplusia ni to jasmonate-mediated induced defense in tomato. J Chem Ecol 36(5):490–499
Simons L, BultmanTL Sullivan T (2008) Effects of methyl jasmonate and an endophytic fungus on plant resistance to insect herbivores. J Chem Ecol 34(12):1511–1517
Stout MJ, Brovont RA, Duffey SS (1998) Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J Chem Ecol 24(6):945–963
Tan CW, Lo JC, Yadav J et al (2011) Methyl jasmonate induced responses in four plant species and its effect on Spodoptera litura fab. performance. J Asia-Pac Entomol 14(3):263–269
Tang F, Fu YY, Ye JR (2015) The effect of methyl salicylate on the induction of direct and indirect plant defense mechanisms in poplar (Populus × euramericana ‘Nanlin 895’). J Plant Interact 10(1):93–100
Thaler JS, Stout MJ, Karban R et al (1996) Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J Chem Ecol 22(10):1767–1781
Thaler JS, Stout MJ, Karban R et al (2001) Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecol Entomol 26(3):312–324
Turlings TC, Loughrin JH, Mccall PJ et al (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci 92(10):4169–4174
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv Insect Physiol 5:229–288
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19(2):195–216
Walters D, Heil M (2007) Costs and trade-offs associated with induced resistance. Physiol Mol Plant Pathol 71(1–3):3–17
Wan Z, Li Y, Liu M et al (2015) Natural infectious behavior of the urediniospores of Melampsora larici-populina on poplar leaves. J For Res 26(1):225–231
Wasternack C, Parthier B (1997) Jasmonate-signalled plant gene expression. Trends Plant Sci 2(8):302–307
Weinhold LC, Ahmad S, Pardini RS (1990) Insect glutathione S-transferase: a predictor of allelochemical and oxidative stress. Comp Biochem Physiol B 95:355–363
Williams L, Rodriguez-Saona C, Castle SC et al (2008) EAG-active herbivore-induced plant volatiles modify behavioral responses and host attack by an egg parasitoid. J Chem Ecol 34(9):1190–1201
Yang F, Zhang Y, Huang Q et al (2015) Analysis of key genes of jasmonic acid mediated signal pathway for defense against insect damages by comparative transcriptome sequencing. Sci Rep 5:16500
Zhang YT, Zhang YL, Chen SX et al (2015) Proteomics of methyl jasmonate induced defense response in maize leaves against Asian corn borer. BMC Genom 16(1):224
Zhu-Salzman K, Zeng R (2015) Insect response to plant defensive protease inhibitors. Annu Rev Entomol 60:233–252
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province(BK20131421) and the Priority Academic Program Development (PAPD) of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest. All of the authors agree to submit this paper. The manuscript has not been previously published in any language anywhere and it is not under simultaneous consideration or in press by another journal.
Additional information
Handling Editor: William B. Walker III.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tianzi, G., Congcong, Z., Changyu, C. et al. Effects of exogenous methyl jasmonate-induced resistance in Populus × euramericana ‘Nanlin895’ on the performance and metabolic enzyme activities of Clostera anachoreta . Arthropod-Plant Interactions 12, 247–255 (2018). https://doi.org/10.1007/s11829-017-9564-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9564-y